Lynn Rolland Hovda, Consulting Editor Poison cases in large animals occur less often than other species but are usually more time consuming and frustrating to manage. Generally, an animal is found dead in the stall or pasture or a sudden outbreak of illness occurs in a herd of animals. A rapid response is necessary, especially in herd cases, to eliminate the toxicant, treat affected animals appropriately, and prevent the development of further cases. The initial history is frequently limited, yet owners, trainers, barn managers, elevator owners, and others may demand an immediate answer. Emotions may run high, especially with the death of a beloved horse or when legal and media related issues arise. The veterinarian is left to provide answers to many complicated issues while attempting to undertake a diagnostic investigation. Finding a toxicant does not necessarily mean it is the causative agent or that it is even related to the problem at hand. Concluding thus without further evidence could skew the investigation and result in an inaccurate diagnosis and further illness or death. A complete toxicologic investigation, including a thorough history, clinical signs, clinicopathologic testing (including necropsy if available), and analytic testing, should be undertaken in a careful and systemic manner. Internet websites can be used to provide accurate and rapid sources of toxicologic information to aid in the investigation and provide treatment options1,2 (Table 54-1). An established working relationship with a diagnostic laboratory providing advanced testing and a board-certified toxicologist to help interpret results will increase the likelihood of a positive diagnosis. TABLE 54-1 Common Internet Websites Refer to references 1 and 2 for a more complete listing of various forms of toxicology information resources. An accurate history is the starting point of any toxicologic investigation and, done correctly, ensures that you have obtained the appropriate samples for further analysis. The investigation may vary from simple to complex but should always be done in a calm and ordered manner so that nothing is overlooked. The history should be used to provide information on circumstances surrounding the problem and may or may not identify the toxicant itself. Caution is advised in that the presence of a toxicant in the environment does not necessarily mean that it is the source of the problem. Finding a toxicant is simply a starting point. Many other factors, including the dose and degree of exposure, need to be considered because exposure to a nontoxic dose generally does not result in a worthwhile diagnosis. Initial questions, however, often revolve around whether a known toxicant is present in the animals’ environment. There are literally hundreds of toxicants, but many people, when questioned, often know which one may be playing a role. Common questions include whether or not medications, pesticides, herbicides, or fertilizers have recently been used or are present on the property. Is rodent control used in barns and outbuildings? Are there old junk piles on the property? What farm equipment/automotive products have been used recently? Have any old buildings been moved or torn down? Are there any known poisonous plants on the property? Recent changes in animal management should be investigated in detail. Animal handlers should be questioned about the introduction of any new feeds, either new bags or fresh batches from a feed mill, or a complete change in feed. Feed should be examined closely for mold and the presence of any foreign bodies. Samples of feed, both as purchased and as fed, should be obtained and stored for further analysis. Hay and pasture should be examined for the presence of blister beetles, clippings from plants such as yew or oleander, other poisonous plants and weeds, foreign bodies, and mold. Again, samples should be taken and saved for analysis. Water samples from the original source and at the trough or stock tank should be taken and conserved in preserving jars for analysis if needed. Species, breed, age, sex, body weight, and body condition are important pieces of information to be collected for all involved animals. Current and past medications used, vaccination history, reproductive status, and illnesses in other animals are other pieces of data that may be useful. Species identification is particularly important because some toxins are species specific or exhibit different toxicologic profiles. In addition, absorption of some toxins in ruminants, horses, and swine varies due to their specific gastrointestinal tracts. Some individual breeds may have different metabolic pathways resulting in delayed metabolism and excretion. The presence of a P-glycoprotein transporter in the MRD-1 gene was recently identified in horses and in the future may play a role in the development of breed-related toxicosis.3,4 Very young and very old animals are often at increased risk for developing signs of poisoning at much lower doses. Neonatal animals absorb some metals and chemicals more readily than adults, and nursing animals may receive toxic substances in the milk. Geriatric animals often have underlying deficiencies in liver metabolism and renal excretion that affect their ability to clear potential toxicants in a timely manner. Live animals should have a complete physical examination performed and be closely examined for the presence of any unusual clinical signs. Ultimately, the conclusion of the physical examination may help narrow the list of toxicants. Both the nature of the signs and sequence of events should be documented. Specific abnormalities such as the color and consistency of mucous membranes, mouth lesions, blistering on pink skin areas, loss of hair around the eyes or tail, horizontal lines on hoof walls, and other abnormal findings may help determine whether the process is specific to an organ system or more generalized in nature. It is important to remember that in some instances this may not be an intoxication because other diseases and/or illnesses may result in similar generalized, nonspecific clinical signs. The time frame from when the animal(s) was last noted to be normal to the onset of signs should be determined, as well as cessation, continuation, and/or duration of signs. Much of this information may need to be obtained in a historical manner from the animal caregiver because the veterinarian is often examining the animal at a single time point in the progression of signs and may otherwise miss valuable information. The results of clinicopathologic tests often shorten the list of potential toxicants and may point toward a specific organ system and/or toxicant. It is invaluable to obtain and either perform or store appropriate samples at the time of the physical examination or postmortem. Blood should be examined for electrolytes, complete blood cell count (CBC), liver enzymes, blood urine nitrogen, and creatinine. Urine should be examined for specific gravity and the presence of crystals, casts, red blood cells, hemoglobin, and methemoglobin. Other body fluids (ocular, cerebrospinal, etc.), hair, and ingesta should be obtained as needed. All samples except the blood and central nervous system (CNS) fluid can be frozen.5,6 Blood should be centrifuged, the clot removed, and the sample stored in the refrigerator. It is important to remember that zinc and many of the trace minerals must be collected in a special tube for analysis.5 If at all possible, animals should be referred to the appropriate diagnostic laboratory for a postmortem completed by a board-certified pathologist. This is especially important if the animal is insured or legal issues are looming on the horizon. Animals sent to the diagnostic laboratory should be identified by tattoo, microchip, ear tag, or other means. Digital pictures taken before shipment can be used as a further source of identification. If a field necropsy is performed, extra samples should be taken because it is far wiser to take more samples than needed and discard them later rather than not have them at all. Often necropsy results will show specific lesions that may confirm a working diagnosis or point the veterinarian in another direction. Sometimes, the postmortem shows only generalized lesions or no lesions at all. Analytic testing can be used to provide a definitive answer, but it must be used correctly and the results analyzed judiciously. Samples should not be sent to an analytic laboratory without some knowledge and forethought. The analytic laboratory will need guidance in terms of what they are testing for, as well as a history including the time since intoxication, dose of toxicant, and postmortem changes. Urine is the gold standard, especially for drug identification, but serum and plasma can also be used for analysis. Different forms of instrumentation/analyses are used for specific substances. For example, metals such as lead, zinc, arsenic, and others are analyzed with atomic absorption and spectrometry.5 Enzyme-linked immunosorbent assay screens are generally sensitive, but the risk of cross-reaction with other substances may be large.6 Drugs, many pesticides, and plant toxins are analyzed using gas or liquid chromatography/mass spectrometry, but unless specifically requested, the results are generally not quantitated and only demonstrate the presence of a toxicant.5,6 In summary, poisoning cases can vary from simple and direct to highly complex. It is important to investigate each one using a systematic approach, including history, clinical signs, clinicopathologic findings, and the results of analytic testing. As the veterinarian works through the investigation, each step may narrow the list of toxicants until a final answer is found. Alternatively, some investigations do not come to a final conclusion and the veterinarian is left to wonder what actually occurred. In many of these cases, environmental and management changes eliminated the toxicant before the investigation, leaving only the aftereffects. Julia H. Wilson Poisonous plants pose significant dangers to all large animal species and should be weighted more highly on a differential list when the animal’s or herd’s history and location suggest potential access. A search for an incentive to have consumed a toxic plant should also occur, unless the toxic plant is palatable, was inadvertently harvested in hay, or its seeds contaminated grain stores. Observation that multiple animals on the premises are affected points to a common source for illness such as a toxicity, feed error, or infectious disease. Plant poisonings are most common in hungry animals at pasture that are driven to consume plants that are normally avoided. The need for forage and therefore risk of toxicosis in both ruminants and equidae is well established. Significant economic losses have been attributed to poisonous plants in regions and countries that rely on grasslands for raising livestock. Studies in the western United States suggest that plant poisonings adversely impact 3% to 5% of rangeland cattle, sheep, goats, and horses.1 In China, poisonous grassland species of plants are responsible for an estimated $12.3 million of all livestock losses, including loss of performance and mortality from 2000-2006.2 The estimated price of plant poisonings in Australia is $100 million (Australian) per year.3 Deaths attributed to toxic plants are estimated to constitute 10% to 14% of all cattle mortality in the major cattle-raising region of Brazil.4 Swine raised in confinement are least likely to be affected by plant poisoning; however, free-roaming swine may consume both garden and pasture species with known toxic potentials. Circumstances that should stimulate investigation of possible plant poisoning on the affected premises include limited availability of forage as evidenced by animal observation and body condition scores; drought affecting plant growth, availability, and potentially high concentrations of toxins such as nitrates; recent storms that may have downed trees or fences; feed changes; inappropriate disposal of garden, shrub, or tree trimmings; and administration of herbal products for medicinal value. The level of toxins within a given plant species is often variable, even in samples from within the same pasture. Toxicity may be more pronounced in specific growth phases of the plant species, during times of stress or rapid growth, or after drying. Endophyte or fungal infections of the plant may be important as well, either as the sole or additional source of toxicity. These factors can be important in the timing and choice of plant specimens to identify, as well as assay for toxicity. The distribution of clinical signs due to plant poisonings may be more difficult to decipher within a group of animals because individual factors play a role in both quantity consumed and expression of clinical signs. More than one type of toxic plant may have been ingested, which may lead to a wider variety of clinical signs. An individual animal may develop a taste for a plant that is avoided by others in their herd or flock or be forced to eat it due to feed competition and low social order. Herbicide application may also enhance the palatability of plant species such as water hemlock.5 Recent fertilizer application may increase nitrate concentrations in both plants and water supplies. Some plants can create a wide range of clinical signs depending on the amount consumed acutely or chronically. Research done on specific toxins and plants, and applied to feeding trials, has demonstrated that differences in metabolism, gastrointestinal flora, and genetics may have a role in disease expression. Yet another variable is interaction between plant toxins and medications. Consumption of hay with two species of nightshade (Solanum spp.) has recently been identified as a factor in the development of clinical signs of ivermectin toxicosis in horses.6 Any suspicion of plant poisoning in an individual or group of individuals in a herd or flock should trigger a methodical investigation. The first step is a complete history of the affected individual(s), herd or flock, and farm. In addition to routine health questions, focus must be placed on current feed and any changes that have been instituted, weather factors that may have affected feed and water access or quality, fertilizer use on forage sources, differences between management of affected and unaffected animals, and the potential for any feeding errors or inadvertent exposure to garden refuse.7 If the history yields evidence to strengthen the suspicion of plant poisoning, immediate removal of access to the suspected source of clinical problems should ensue. If multiple animals have been affected over time, establishment of a time line of events and cases may be a useful way to evaluate the history and determine the cause. The second step in the investigation should be thorough examination of affected animals to identify and characterize clinical abnormalities and lesions. Low body condition scores, particularly if accompanied by a strong appetite, should raise clinical suspicion of consumption of normally unpalatable plants. Pregnant animals should be evaluated for signs of potential abortion. Quick access to an animal poison control center or available online database where clinical signs are entered to yield potential diagnoses may help veterinarians quickly sort through potential differential diagnoses for the animal(s).8 Symptomatic treatment is often the first line of medical response if the etiology has not been determined or a specific antidote is not available or approved for use in that species. Stress of treatment, particularly for ruminants, must be weighed against the benefits. Activated charcoal administration remains advisable for most acute toxicoses if the animal can withstand the stress of administration. In ruminants, rumenotomy for evacuation of contents and instillation of activated charcoal should be considered. In monogastrics, stomach lavage followed by instillation of a slurry of activated charcoal in water may be the best option for acute cases. A limited number of specific antidotes are described for a few plant poisonings, but their use may be restricted by cost and the need to avoid food residues. Enhanced excretion of toxins through the use of laxatives or manipulation of urine or rumen pH may be applicable in specific situations where the toxic plant or principle has been confirmed. Postmortem examination of affected animals should take place as soon after death as possible to maximize the opportunities to identify lesions and an etiology. In some plant poisonings, a specific odor may be noted, such as a bitter almond smell in cyanide poisoning (e.g., Prunus spp.), acetone smell with white snakeroot poisoning, and a mousy aroma due to poison hemlock ingestion.5 Samples should be taken of rumen or stomach contents to look grossly and microscopically for recognizable plant leaf fragments or seeds. An aliquot of the ingesta should be labeled and frozen as soon as possible if toxicologic analysis is anticipated. Depending on the history time line, more ingesta samples may be warranted from other segments of the digestive tract. Intact seeds may be identifiable in the distal intestinal tract or manure. Samples of aqueous humor, amniotic fluid, and fetal stomach fluid for nitrate concentrations should be considered if clinical signs are compatible with that etiology (see later). If the animal has died within a few hours, blood samples for hematologic, chemistry, and toxin profiling can be rewarding. Tissue samples are important for characterizing histologic lesions to confirm a plant toxicity or alternative etiology. All grossly affected tissues should be sampled and a piece of liver frozen for toxicologic analysis. If neurologic signs were observed, the brain should be removed with proper barrier precautions.7 Identification of the plants, feedstuffs, or water responsible for clinical signs or death(s) is important for guiding treatment and further control. This may entail feed inspection or walking the pastures and fence lines to look for not only feasible plants that fit the clinical signs but also evidence that it has been consumed. If blue-green algae poisoning is a potential etiology, this tour must include inspection of water sources, where a bloom of blue-green algae may be found to be responsible for sudden death (see next section on Blue-Green Algae). The veterinarian, farm manager, or owner should visually inspect feedstuffs, looking for not only noxious plant or seed contaminants but also for mycotoxins (see section on Mycotoxins). Unknown plants should be photographed and placed in plastic bags for later identification and potential toxin determination. If identification will be delayed, plant samples should be pressed between leaves of brown paper and weighted down with heavy books for several days; an aliquot should be frozen for later analysis if indicated. For identified plants known to have variable toxicity, such as larkspur (Color Plate 54-1) or selenium-accumulating plants, multiple samples should be taken of the same species, concentrating on areas where the plants have been visibly browsed or grazed.9 Resources for plant identification include toxicology texts, the U.S. Department of Agriculture (USDA) plant identification website, the USDA Poisonous Plant Research Laboratory website and its publications, other country-specific websites, and university or extension service plant experts.10–16 In the United States, plant samples can be sent to the USDA Poisonous Plant Research Laboratory or Texas A&M’s Veterinary Diagnostic Laboratory. Common plant poisonings often trigger a recognizable pattern of clinical signs and disease progression. These are reviewed below in more detail, with emphasis on diagnostic features, treatment options, and preventive measures. Less frequent causes of livestock morbidity and mortality are listed in tables, grouped by predominant clinical signs. Highlights of newly described toxicities that have the potential to harm multiple animals are also presented in this chapter section. Poisonous plants, chemical toxins, lightning strikes, and infectious diseases are causes of sudden death that should be considered when investigating sudden death in multiple animals. Numerous plant species have the potential to kill multiple animals within minutes to hours (Table 54-2). TABLE 54-2 Plants with Potential to Cause Sudden Death in Large Animals* * Plant names in bold are discussed in more detail in the text. † Species listed in relative order of toxicity importance: B, Bovine; C, caprine; E, equine; O, ovine, P, porcine.
Disorders Caused by Toxicants

Type
Organization
Web Address
Animal poison control centers
ASPCA Poison Center
www.aspca.org/pet-care/animal-poison-control
Pet Poison Helpline
www.petpoisonhelpline.com
Diagnostic laboratories
American Association of Veterinary Laboratory Diagnosticians
www.aavld.org
General information
Google Scholar
www.scholar.google.com
Veterinary Information Network
www.vin.com
Literature search sites
International Veterinary Information Service
www.ivis.org
Medline
http://medlineplus.gov/
National Agriculture Library
http://agricola.nal.usda.gov
Pub Med
www.ncbi.nlm.nih.gov/pubmed/medline.html
Toxicology information
Extoxnet
http://extoxnet.orst.edu
InChem
www.inchem.org
MSDS Search Site
http://www.msdssearch.com/
Toxnet
http://www.toxnet.nlm.nih.gov/
History
Clinical Signs
Clinicopathologic Testing
Analytic Testing
Poisonous Plants
Sudden Death
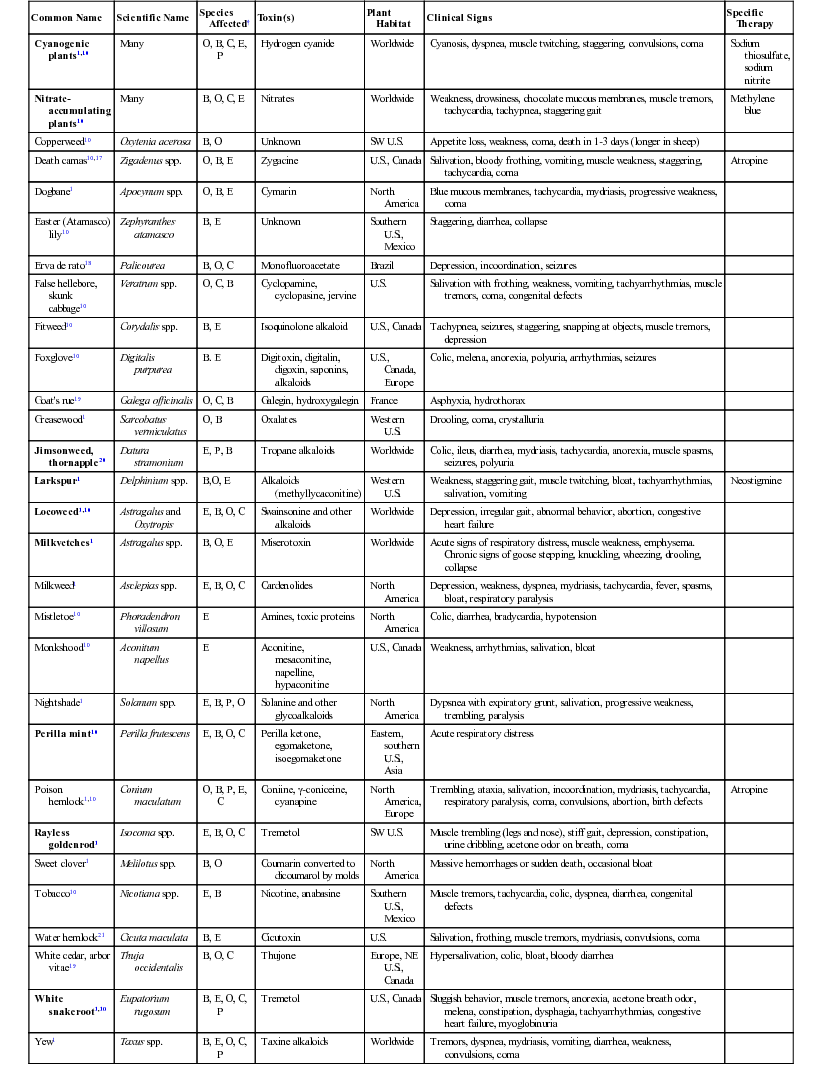
Common Name
Scientific Name
Species Affected†
Toxin(s)
Plant Habitat
Clinical Signs
Specific Therapy
Cyanogenic plants1,10
Many
O, B, C, E, P
Hydrogen cyanide
Worldwide
Cyanosis, dyspnea, muscle twitching, staggering, convulsions, coma
Sodium thiosulfate, sodium nitrite
Nitrate-accumulating plants10
Many
B, O, C, E
Nitrates
Worldwide
Weakness, drowsiness, chocolate mucous membranes, muscle tremors, tachycardia, tachypnea, staggering gait
Methylene blue
Copperweed10
Oxytenia acerosa
B, O
Unknown
SW U.S.
Appetite loss, weakness, coma, death in 1-3 days (longer in sheep)
Death camas10,17
Zigadenus spp.
O, B, E
Zygacine
U.S., Canada
Salivation, bloody frothing, vomiting, muscle weakness, staggering, tachycardia, coma
Atropine
Dogbane1
Apocynum spp.
O, B, E
Cymarin
North America
Blue mucous membranes, tachycardia, mydriasis, progressive weakness, coma
Easter (Atamasco) lily10
Zephyranthes atamasco
B, E
Unknown
Southern U.S., Mexico
Staggering, diarrhea, collapse
Erva de rato18
Palicourea
B, O, C
Monofluoroacetate
Brazil
Depression, incoordination, seizures
False hellebore, skunk cabbage10
Veratrum spp.
O, C, B
Cyclopamine, cyclopasine, jervine
U.S.
Salivation with frothing, weakness, vomiting, tachyarrhythmias, muscle tremors, coma, congenital defects
Fitweed10
Corydalis spp.
B, E
Isoquinolone alkaloid
U.S., Canada
Tachypnea, seizures, staggering, snapping at objects, muscle tremors, depression
Foxglove10
Digitalis purpurea
B. E
Digitoxin, digitalin, digoxin, saponins, alkaloids
U.S., Canada, Europe
Colic, melena, anorexia, polyuria, arrhythmias, seizures
Goat’s rue19
Galega officinalis
O, C, B
Galegin, hydroxygalegin
France
Asphyxia, hydrothorax
Greasewood1
Sarcobatus vermiculatus
O, B
Oxalates
Western U.S.
Drooling, coma, crystalluria
Jimsonweed, thornapple20
Datura stramonium
E, P, B
Tropane alkaloids
Worldwide
Colic, ileus, diarrhea, mydriasis, tachycardia, anorexia, muscle spasms, seizures, polyuria
Larkspur1
Delphinium spp.
B,O, E
Alkaloids (methyllycaconitine)
Western U.S.
Weakness, staggering gait, muscle twitching, bloat, tachyarrhythmias, salivation, vomiting
Neostigmine
Locoweed1,10
Astragalus and Oxytropis
E, B, O, C
Swainsonine and other alkaloids
Worldwide
Depression, irregular gait, abnormal behavior, abortion, congestive heart failure
Milkvetches1
Astragalus spp.
B, O, E
Miserotoxin
Worldwide
Acute signs of respiratory distress, muscle weakness, emphysema. Chronic signs of goose stepping, knuckling, wheezing, drooling, collapse
Milkweed1
Asclepias spp.
E, B, O, C
Cardenolides
North America
Depression, weakness, dyspnea, mydriasis, tachycardia, fever, spasms, bloat, respiratory paralysis
Mistletoe10
Phoradendron villosum
E
Amines, toxic proteins
North America
Colic, diarrhea, bradycardia, hypotension
Monkshood10
Aconitum napellus
E
Aconitine, mesaconitine, napelline, hypaconitine
U.S., Canada
Weakness, arrhythmias, salivation, bloat
Nightshade1
Solanum spp.
E, B, P, O
Solanine and other glycoalkaloids
North America
Dypsnea with expiratory grunt, salivation, progressive weakness, trembling, paralysis
Perilla mint10
Perilla frutescens
E, B, O, C
Perilla ketone, egomaketone, isoegomaketone
Eastern, southern U.S., Asia
Acute respiratory distress
Poison hemlock1,10
Conium maculatum
O, B, P, E, C
Coniine, γ-coniceine, cyanapine
North America, Europe
Trembling, ataxia, salivation, incoordination, mydriasis, tachycardia, respiratory paralysis, coma, convulsions, abortion, birth defects
Atropine
Rayless goldenrod1
Isocoma spp.
E, B, O, C
Tremetol
SW U.S.
Muscle trembling (legs and nose), stiff gait, depression, constipation, urine dribbling, acetone odor on breath, coma
Sweet clover1
Melilotus spp.
B, O
Coumarin converted to dicoumarol by molds
North America
Massive hemorrhages or sudden death, occasional bloat
Tobacco10
Nicotiana spp.
E, B
Nicotine, anabasine
Southern U.S., Mexico
Muscle tremors, tachycardia, colic, dyspnea, diarrhea, congenital defects
Water hemlock21
Cicuta maculata
B, E
Cicutoxin
U.S.
Salivation, frothing, muscle tremors, mydriasis, convulsions, coma
White cedar, arbor vitae19
Thuja occidentalis
B, O, C
Thujone
Europe, NE U.S., Canada
Hypersalivation, colic, bloat, bloody diarrhea
White snakeroot1,10
Eupatorium rugosum
B, E, O, C, P
Tremetol
U.S., Canada
Sluggish behavior, muscle tremors, anorexia, acetone breath odor, melena, constipation, dysphagia, tachyarrhythmias, congestive heart failure, myoglobinuria
Yew1
Taxus spp.
B, E, O, C, P
Taxine alkaloids
Worldwide
Tremors, dyspnea, mydriasis, vomiting, diarrhea, weakness, convulsions, coma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


