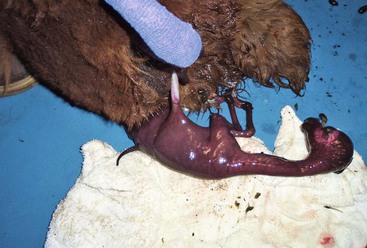
Disorders and Diseases of Pregnancy
Under proper management systems with good nutrition and veterinary supervision, the annual pregnancy rate is estimated to be 78%, with 7% to 12% of pregnancies lost between conception and parturition.1,2 In South America, under less optimal traditional management systems, the birthing rate may be as low as 45%.1,2 Pregnancy losses may occur on an individual basis or on a herd basis and may be caused by infectious or noninfectious factors. This chapter discusses pregnancy loss in alpacas and llamas, as well as other diseases related to pregnancy. Uterine torsion is a common complication of late gestation, for which the producer and practitioner should have an emergency plan in place.
Pregnancy Loss
A study of 158 pregnancies in alpacas in New Zealand reported a 25.7% pregnancy loss rate after 30 days of gestation and 9.6% to 16.7% losses occurring after day 120 of gestation.3 In autumn-bred females, 17.3% of pregnancy losses occurred before 81 days of gestation, whereas spring-bred females had a loss rate of only 2.8% in the same gestational period.3 Seasonal effects were not seen in other groups on a neighboring farm. In this particular study, most of the animals had been imported from Chile, and several of these animals experienced recurrent pregnancy loss over several seasons. Serial serum progesterone determination in pregnant females demonstrated a decrease to baseline of progesterone occurring simultaneously with the loss of the pregnancy in eight animals.3
Early pregnancy loss is defined as loss of the embryo or fetus before 50 days of gestation. Frequently, no outward signs of loss are evident. However, in pregnancies beyond 35 days, small amounts of tissue or fluid may be evident, especially around the dung pile. In a large proportion of cases, the producer may suspect that the female is open because she either fails to develop abdominal distention in late gestation or may cush or be receptive in the presence of mating animals or a solitary male over a fence line. However, by the time the owner suspects that the pregnancy has been lost, it is often too late to evaluate the female clinically and reach a diagnosis. This is why pregnancy should be monitored through several examinations in the first 60 to 90 days. In our practice, we recommend pregnancy evaluations at 14, 25 to 30, and 45 to 60 days. If the female has had a history of recurrent pregnancy loss or infertility, particularly because of endometritis, another evaluation is scheduled between 80 and 90 days. This schedule allows us to monitor the pregnancy until the time when signs of fetal loss can be seen. It is also our recommendation that the females not be moved from the breeding farm during this period. During the early period, ultrasonographic signs of impending pregnancy loss include smaller-than-normal embryonic vesicle, echogenic spot (speckling) of the embryonic vesicle, absence of fetal heart beat after 25 days, poor definition of fetal structures, increased echogenicity of fetal fluid, slow growth in size, echogenic ring within the vesicle, disorganized membranes and collapsed amnion, and increased uterine edema and endometrial folds (Figure 23-1).
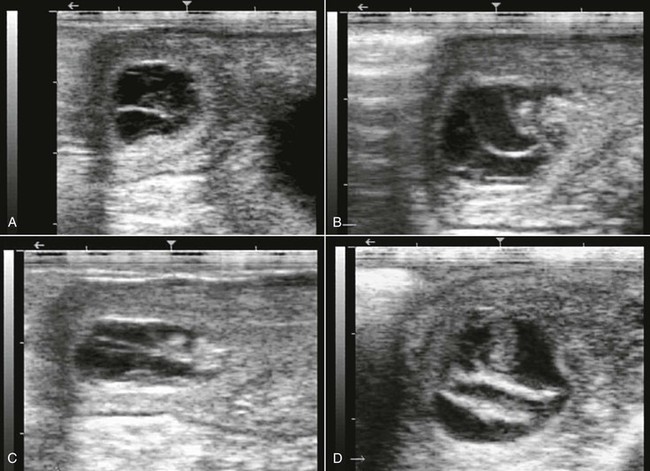
Figure 23-1 Ultrasonograms of Early Pregnancy Loss in Alpacas.
A, 27 days pregnancy with disorganized embryonic vesicle no fetal heart beat. B, 35 days pregnancy with collapsed vesicle. Note increased echogenicity of fluid. C, Same case in B, one day later. D, 45 days pregnancy with collapsed vesicle. Note the dense fetal membranes.
Pregnancy losses after the first trimester result in visible signs of expulsion of the fetus and its membranes. The producer may notice vaginal discharge in the pregnant female, in which case careful examination of the pasture or pen may yield the abortus. Late pregnancy losses may be preceded by clinical signs such as mammary gland development, restlessness, and colic. In many cases, the producer may find the abortus in a pasture of pregnant females and will have to identify which female lost the pregnancy. In either case, the animal which aborted must be separated from the herd and the aborted fetus and tissues collected to be submitted for laboratory investigations. As the aborted material may be infectious, the remaining pregnant females should be either moved to a clean pasture or contained in the area where the abortus was discovered with a temporary fence erected around it. If it is unknown which pregnant female aborted, all the population of females at risk may need to be reevaluated by using ultrasonography. This is also very important in case of an abortion outbreak so that some of the females in the process of aborting may be identified sooner. Owners should also be warned about the zoonotic risk of many of the abortion-causing agents.
General Approach to Diagnosis of Pregnancy Loss or Abortion
Examination of the female should include a thorough physical examination. Young females bred before they achieved 63% of adult weight and height have a higher risk of pregnancy loss because of immaturity. Females with poor body condition or severe weight loss may also experience pregnancy loss, particularly in harsh winters (Figure 23-2). Serum may be collected for trace mineral assay, as deficiencies or excess may cause fetal loss. Ultrasonographic examination of the uterus may demonstrate fluid or membrane retention. Uterine culture should be performed using a double-guarded swab and an appropriate culture medium to transport the specimen to the laboratory. A vaginal examination will demonstrate any cervical damage or vaginal lacerations that commonly occur in late-term abortion because sufficient cervical dilation often does not occur before fetal expulsion. Endometrial biopsy is highly recommended in females with recurrent pregnancy loss.
The placenta should be evaluated for signs of inflammation, completeness, and any other abnormalities. A more detailed account on the examination of the placenta is given elsewhere in this text. Areas of reduced or absent chorionic villi may correspond to areas of uterine fibrosis or presence of twins. Histopathologically, evidence of inflammation, infection, or placental insufficiency may be identified. If the placenta cannot be submitted in its entirety, samples should be obtained from the cervical region, the body of the uterus, and the base, middle, and tip of each horn, as well as from any abnormal-looking area. Samples from lesions should have wide margins, including both normal and abnormal looking areas. Samples should be taken in duplicates and submitted fresh on ice and fixed in 10% formalin (Figure 23-3). Particular attention should be given to the number of placentas and the anatomy of the umbilical cord. Impression smear from the chorionic surface are often very helpful in the diagnosis of bacterial or fungal placentitis.
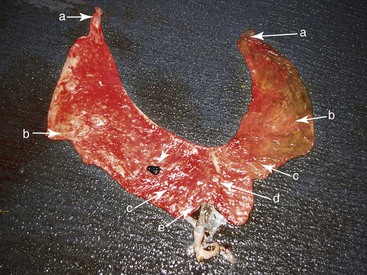
Figure 23-3 Late-Term Placenta Showing Areas to Sample for Histopathology.
A, Tip of the uterine horns. B, Middle of the uterine horns (avoid the medial areas adjacent to the bifurcation). C, Base of the uterine horns. D, Body of the uterus. E, Cervical star. Nonlabeled arrow points to a hippomane (normal).
The fetus should ideally be submitted in its entirety to the diagnostic laboratory. Evaluation of the aborted fetus should include weight measurements (crown–rump length) to estimate gestational age and rule out intrauterine growth retardation. Formulas for the estimation of gestational age are available but are not always accurate (Table 23-1). Examination of the surface of the body may reveal lesions suggesting bacteriologic examination. External examination and necropsy should be very thorough and may reveal severe developmental abnormalities or evidence of infection. Fluid should be aspirated aseptically from the pleural and abdominal cavities as well as from the fetal stomach and submitted for microbiologic analysis. Tissue samples from any grossly abnormal areas and from all major organs (i.e., liver, lung, kidney, adrenal gland, placenta, heart-thymus, brain, spleen, and small intestine) should be taken for histopathology and immunohistochemistry.4 Cardiac blood and frozen sections of the liver and brain should be provided for toxicologic studies.
TABLE 23-1
Formulas for the Estimation of Gestational Age (GA) in Days from Fetal Measurements in Alpacas and Llamas
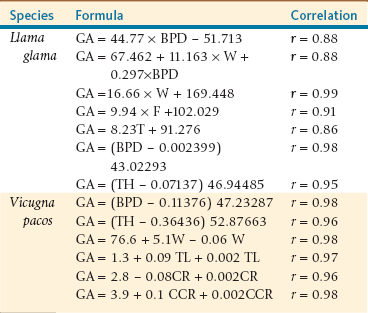
Adapted from Tibary A, et al: Infectious causes of reproductive loss in camelids, Theriogenology 66:633-647, 2006.
Etiology
The etiology of abortion is often very difficult to determine and is often frustrating for the owner and the practitioner. The diagnosis rate rarely reaches 30% of all submissions.5 This may be attributed to lack of sufficient clinical and laboratory data and lack of expertise in camelid pathology. As in other species, causes of abortion in alpacas and llamas fall into distinct categories: infectious and noninfectious. Noninfectious causes of abortion may be sporadic or affect several animals in a herd, as in the case of abortions caused by nutritional deficiencies or administration of some drugs. Infectious abortions are further subdivided into the types of organisms responsible: viruses, bacteria, protozoa, or fungi. Noninfectious causes of abortion may be categorized as maternal causes, fetal causes, nutritional causes, and iatrogenic causes. The following sections will detail what is known about these causes of pregnancy loss in alpacas and llamas.
Infectious Causes of Pregnancy Loss
Viral Causes of Abortion
Viral causes of pregnancy loss are dominated by abortions caused by bovine viral diarrhea virus (BVDV). It was long hypothesized that camelids were more resistant or less susceptible to pestiviral infections compared with domestic ruminants. However, clinical reports and experimental studies in recent years showed that BVDV may represent an emergent and important disease in these species. Growing evidence in the literature suggests that BVDV may be responsible for reproductive wastage in camelids. The most common serotype that affects alpacas and llamas is noncytopathic BVDV-1b.6 Pestiviral infection may manifest as one of several clinical syndromes. Initially, an animal is infected by the virus through exposure to an infected animal; it is hypothesized that BVDV was introduced into camelid populations from contact with infected cattle. Seroconversion and virus shedding occurs, and the animal either shows signs of illness or maintains subclinical infection. Clinical infection is manifested by immunosuppression and subsequent secondary bacterial or viral infections. Commonly affected systems include the respiratory, gastrointestinal, and lymphatic systems, although diarrhea does not seem to be a component of disease in alpacas or llamas. Subclinical infection may not be apparent besides transient fever, inappetance, lethargy, and changes in fleece quality.7
If the infected animal is pregnant, the fetus may be affected in one of several ways. In early gestation, early embryonic death may occur; or in cattle, congenital malformations such as cerebellar hypoplasia, hydrocephalus, microphthalmia, and various other craniofacial, neural, and ocular defects have been reported.8 No congenital defects due to infection with BVDV have been reported in alpacas or llamas.9 Births of persistently infected crias have been documented.7,10,11 In cattle, it is known that infection of the pregnant cow before 125 days of pregnancy results in the birth of a persistently infected (PI) animal. This period of immuno-naivety in the pregnancy of alpacas or llamas is not currently known. However, a study of an outbreak on a farm showed that the transplacental infection rate was 82%, resulting in the birth of a PI cria in 70% of the cases (n = 10) when pregnant females were exposed between 64 and 114 days of pregnancy.12 Because of the great variability of pregnancy length, determination of the exact period of susceptibility is very costly. However, on the basis of available information, it may be assumed that this period ranges between 45 and 120 days of pregnancy. During this stage, the fetus is not immunocompetent, and infection by the virus is not recognized by the fetus as non-self; thus, no antibodies to the virus are ever formed. The fetus then becomes tolerant to the infection, and if it survives, the neonate then serves as a source of viral shedding in the herd.11,13 The final clinical picture is a female that becomes infected late in gestation when the fetus has developed a functional immune system. In these cases, the fetus will mount an antibody response, seroconvert, and usually recover along with the dam. Abortion may occur at any stage of gestation or a weak PI cria may be born prematurely.
The birth of a PI animal has significant effects on a herd of animals. The PI animal itself may appear clinically normal but will continue to shed viral particles or may suffer from ill-thrift and secondary infections.8 In cattle, PI animals may develop full-blown mucosal disease, which is highly fatal; this has not been reported in camelids and crias, which tend to develop chronic disease.9 A recent study has shown that chronically infected and persistently infected crias have poor growth rate and often show anemia and monocytosis. The most common clinical symptoms include chronic wasting, diarrhea, and respiratory diseases. About 50% of the animals will die in the first 6 month of life.12
Of primary importance to prevent or minimize BVDV infection on a farm is to segregate pregnant females from PI crias or transiently infected animals and from any cattle, sheep, or goats to prevent interspecies transmission from positive animals, although the roles of other domestic species in transmission to camelids are not completely elucidated. PI crias have been shown to shed the virus in all body fluid and are an important source of contamination.13 Viral isolates from camelids have not completely matched those that infect other ruminants, and geographic location seems to be implicated in camelid disease in North America, as a majority of BVDV cases in camelids reported no contact of affected animals with cattle or sheep.6,9 It is recommended that producers take precautions with female alpacas or llamas that travel to another farm for breeding along with their suckling cria and develop an isolation protocol for animals that travel for shows. It is recommended that a BVDV screening program be put into effect, with no less than 10% or 15 animals in the herd tested.9 Suckling crias may have circulating antibodies from the dam’s milk or if fed bovine colostrum.9
Research of pestiviral infection in camelids has been focused in three main areas: (1) determining the seropositivity of various herds through epidemiologic studies; (2) clinical examination of affected animals; and (3) experimental inoculation of animals with BVDV. The seropositive rate in epidemiologic studies has been widely variable and demonstrated camelids infected with BVDV types 1a, 1b, and 2, with 1b being the most common.6 Experimental inoculation of pregnant llamas demonstrated seroconversion of the dams but no other clinical effects.14 One experimentally infected llama aborted 5 months after infection, but no BVDV was found in the fetus.14
Diagnosis of BVDV infection in an alpaca or llama is similar to that in cattle. Virus isolation from tissue samples of aborted fetuses may be performed; preferred tissues are blood, lymph nodes, and placenta.15 Immunohistochemistry may be performed on formalin-fixed tissues of an abortus as well.15 Polymerase chain reaction (PCR) may be performed on whole blood samples and is the most common screening method of infection in neonatal crias. Serology of whole blood will provide antibody titers to demonstrate seroconversion, although it will not distinguish active infection from prior exposure. The recommendation following a diagnosis of a PI cria is euthanasia and identification of any other infected animals in the herd to minimize propagation of the disease.
No vaccines for BVDV are licensed for use in alpacas and llamas, although many animals have been administered cattle vaccines without knowledge of their efficacy or safety. One study challenged nonpregnant female alpacas with BVDV after vaccination with a commercial bovine BVDV modified-live vaccine, which included types 1 and 2.16 All animals seroconverted; no vaccinated animals were PCR positive for BVDV after viral inoculation, whereas nonvaccinated controls were PCR positive and developed self-limiting respiratory disease. However, vaccination of alpacas and llamas for BVDV is not recommended given the low prevalence of the disease, the off-label use of vaccines, and unknown efficacy in pregnant animals.
Abortion cases have been reported by owners during a recent outbreak of a viral respiratory disease in alpacas (acute respiratory distress syndrome). The suspected etiology of this syndrome is a coronavirus.17 The disease presents as an influenza-like syndrome progressing to a severe respiratory distress resulting from pulmonary congestion and marked pleural effusion. Abortion is likely caused by severe fetal hypoxia. We have successfully managed two alpaca cases at 10 and 11 months of pregnancy with supportive therapy based on oxygen insufflations, nonsteroidal antiinflammatory drugs (NSAIDs), and broad-spectrum antimicrobials.
Other viruses that have been associated with abortion in camelids include equine herpes virus-1 (EHV-1) and equine arteritis virus (EAV). Although abortion caused by EHV-1 has not been reported in alpacas and llamas, the virus has been isolated from abortion cases in the dromedary. The virus is also known to cause neurologic diseases in alpacas and llamas.18 EAV viral particles were identified in one late-term alpaca abortus by reverse transcriptase PCR.19 Five adult animals in the herd were seropositive for EAV. More recently, blue tongue virus was reported to cause severe clinical syndrome and death in alpacas.20 The exact role of these viruses in alpaca and llama abortion is unknown; however, diagnostic laboratories should be aware of this possibility.
Bacterial Causes of Abortion
Bacterial causes of abortion in alpacas and llamas are the predominant infectious causes of pregnancy loss in these species with several organisms isolated.5
Chlamydophila spp. have been identified as a cause of abortion in llamas.21 Two aborted fetuses and one weak cria, which subsequently died, were positive for Chlamydophila spp. Affected females were seropositive, and nonaffected pregnant females were treated with long-acting oxytetracycline injections, after which no further abortions occurred. In one study of normal vaginal flora in alpacas, no Chlamydophila spp. were identified, which supports the role of Chlamydophila spp., not normal flora, as pathogenic organisms.22 Vaccines licensed for use in sheep have been used off-label in camelids, but their influence on the incidence of abortion is not known. Diagnosis of Chlamydophila psittaci and C. abortus may be made by using PCR on swabs from the placenta and fetal tissues. However, maternal serology is not always helpful.
Brucellosis has been reported to cause abortion in camel herds, but the role of this disease in alpacas and llamas has not been fully investigated.4 Abortion caused by Brucella melitensis has been reported in alpacas in Peru.23 Llamas exposed to B. abortus showed bacteremia and colonization of various tissues. Exposed animals also mounted a humoral antibody response.24 Experimental inoculation of llamas with B. abortus resulted in late gestation abortion in one animal.25 Organisms were disseminated through fetal tissue and placenta. Histologic features were similar to those found in cattle that abort because of infection by the same agent (i.e., subacute placentitis). B. abortus was also isolated from the dam’s mammary and lymph tissues after euthanasia.
Positive titers for Leptospira spp. were found in 47.3% to 96.2% of llamas tested in Argentina.26 Seroprevalence ranged from 0% to 13% in guanacos, and from 9% to 62.8% in vicunas. However, very little evidence exists for the role of these organisms in pregnancy loss in camelid species, although leptospiral organisms are a significant cause of abortion in other domestic large animals, including horses and cattle. The most common isolates were L. interrogans serogroups Icterohaemorrhagiae and Ballum. Another study in Peru demonstrated that 79.24% of tested alpacas were seropositive for the serogroup Pomona and that seroprevalence was associated with the rainy season.27 In a report from Oregon, L. Pomona and L. grippotyphosa were identified as possible causes of abortion on the basis of dam serology.5 Commercial vaccines have been used off-label in camelids with variable results. Several studies have demonstrated highly individual levels of antibody titers and short duration of immunity.28,29 Currently, vaccination is only recommended for animals in endemic areas. Field observations warn against using these vaccines in the first trimester and the last 2 months of pregnancy.
Clinical disease caused by Listeria monocytogenes is manifested in most species as three separate syndromes: (1) meningoencephalitis, (2) septicemia (monogastrics and neonatal ruminants), and (3) abortion in females in late pregnancy.5,30 Infection with L. monocytogenes has been demonstrated in adult and neonatal 31–33 llamas and alpacas; however, only one case of Listeria-induced abortion has been reported.30 A multiparous female aborted at 8 months of pregnancy after a short period of lethargy. L. monocytogenes was cultured from the fetal liver and lung, and diffuse placental lesions were noted on histopathology. A uterine culture of the affected female was obtained but could not be processed by the laboratory. The female became pregnant 30 days after the abortion, and no other animals on the property were affected. The llama was subsequently found to be infected with Mycoplasma hemolamae, which has been associated with immunosuppression. Neonatal cases of septicemia caused by L. monocytogenes are mostly attributed to failure of passive transfer, although negative uterine cultures after parturition are often not available to rule out maternal infection and transplacental infection.32,33
Abortion caused by Campylobacter fetus was reported in alpacas in the United Kingdom.34 Four abortions and one premature nonviable cria were born to females in late pregnancy over a 6-week period. Sheep were present on the farm but were geographically separated and had no abortions that year. Two aborted fetuses were examined. One placenta demonstrated a focal area of necrosis and the other generalized necrotic placentitis. Organisms were isolated from placental tissue as well as from fetal stomach contents. One dam was found to have a swollen tongue with a diphtheritic membrane, which may have been unrelated to Campylobacter infection. Fecal cultures of remaining pregnant alpacas demonstrated the organism in 36% of animals. Four of the five affected females were bred and became pregnant within 31 and 150 days of abortion. In sheep, it has been demonstrated that infection results in protective immunity for several years; it is not known if this occurs in camelids. Differentials for late-term abortion in camelids with placental lesions and exposure to sheep should include C. fetus, although further investigation into this pathogen in these species is warranted. However, it is important to differentiate abortion caused by Campylobacter spp. from those caused by Campylobacter-like organisms such as Arcobacter spp., which has also been isolated from an alpaca abortus.35
Q-fever, caused by Coxiella burnetii, is a well-established cause of abortion in camels and present serious risks to human health.4 To date, the implication of this organism in alpaca and llama abortions has not been reported. More investigation of its role in abortion in these species is warranted.
Abortion caused by M. haemolamae has not been demonstrated in camelids, but in utero transmission from a nonparasitemic dam to an alpaca cria has been reported.36 A 4-day-old cria had almost 100% of erythrocytes affected by Mycoplasma organisms; the cria died despite stabilization attempts. Both cria and dam were PCR positive for M. haemolamae. Transplacental infection of crias should be suspected in addition to failure of passive transfer in critically ill neonates.
Other isolates from cases of alpaca and llama abortion include Escherichia coli, Streptococcus sanguis, Arcanobacterium pyogenes (newly renamed Trueperella pyogenes), and Pseudomonas spp.5,37
Protozoal Causes of Abortion
The most common cause of protozoal abortion in alpacas and llamas is Neospora caninum. It has long been known that the organism is responsible for abortion and congenital infection of cattle. One study in Argentina demonstrated seroprevalence of 4.6% in llamas, whereas a separate study in Peru demonstrated antibodies against N. caninum in 36% of alpacas and 31.5% of llamas.38,39 Investigation of abortion in 15 alpacas and llamas in Peru definitively diagnosed N. caninum in three cases by demonstration of parasitic deoxyribonucleic acid (DNA) in mid- to late-term aborted fetal tissues.40 Another study by the same research group demonstrated that N. caninum infection was associated with 28% of aborted fetuses, with abortions occurring mostly in mid-gestation.41 As the definitive hosts of N. caninum are domestic and wild canids, producers should be advised to monitor and limit exposure of their camelids to these species and their excrement.
Infection and abortion in alpacas and llamas caused by Toxoplasma gondii has been long suspected.21 Studies of adult camelids in South America demonstrated variable seroprevalence of T. gondii antibodies: from 35% to 44.2% in llamas, 16.3% in alpacas, and 5.5% in vicunas.38,39,42 Seroprevalence in llamas in the U.S. Pacific Northwest was 33.5% in one study.43 These epidemiologic studies demonstrated that camelids are intermediate hosts for T. gondii, although parasitic cysts in tissues have not been found, and the effects on pregnancy remain uncharacterized.
One research group examined pregnant llamas infected with T. gondii during pregnancy.44 One animal was experimentally inoculated at 82 days of pregnancy and one animal was naturally infected between days 26 and 119 of pregnancy; both llamas seroconverted and yet remained clinically healthy. Both animals delivered healthy viable crias, and no T. gondii antibodies were found in the serum of the crias prior to colostrum ingestion, which suggests that transplacental transmission did not occur. However, two llamas had rising T. gondii titers and suffered abortion.45 Fetal fluids from one of the aborted crias were positive for T. gondii antibodies. In contrast, a study of 50 aborted fetuses in Peru did not demonstrate any T. gondii DNA in analyzed tissues.41 On the basis of these studies, toxoplasmosis does not appear to be a common cause of abortion in alpacas and llamas; however, producers should minimize the amount of contact between naive camelids and cats, the definitive host of T. gondii, as well as keeping the feed covered.
Sarcocystosis is the most prevalent of protozoal infections worldwide, although its role in pregnancy loss and abortion in camelids has not been fully elucidated. Infection of alpacas and llamas as intermediate hosts has been commonly reported, and development of sarcocysts in tissues leads to condemnation of the meat and severe economic losses in South American countries. In Argentina, the seroprevalence of Sarcocystis aucheniae and S. cruzi were determined, with 96% of samples positive for only one genus and 75% positive for both.38 One report of abortion caused by sarcocystosis in an alpaca demonstrated features of both acute and chronic infection in the female.46 It was hypothesized that abortion was caused by anemia in the dam and endogenous release of prostaglandin F2-alpha (PGF2α) from systemic stress, and the fetus did not show any pathology attributed to parasitic infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree