Chapter 6 Diseases of the Uvea
With the exception of uveitis and neoplasia, abnormalities of the equine uveal tract are relatively uncommon and often of minimal consequence to vision or comfort. However, some innocuous conditions can mimic serious disease, so proper assessment is important to prevent misinterpretation and inappropriate clinical action. This chapter will discuss diseases of the equine uvea, with the exception of equine recurrent uveitis (ERU), which is covered specifically in Chapter 8.
Clinical Anatomy and Physiology
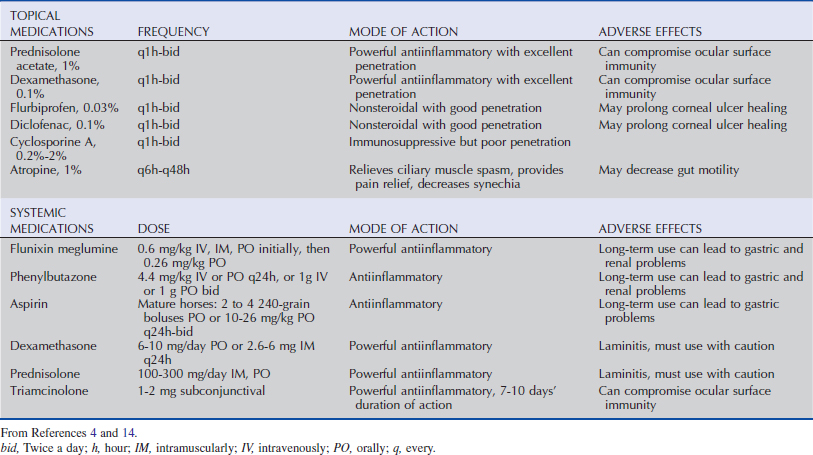
The uveal tract is comprised of three components: the iris, ciliary body, and choroid and is anatomically similar to that of other species in most clinically relevant aspects. As in most herbivores, the adult equine pupil is horizontally oval. The anterior surface of the iris is without a contiguous epithelial layer and is composed of connective tissue interspersed with fibroblasts and melanocytes. Beneath this surface layer is the iris stroma, which consists of chromatophores, fibroblasts, and collagen fibers that cradle a plexus of blood vessels. Within the central stroma and circumferentially oriented along the pupillary margin is the parasympathetically innervated iris sphincter muscle. Immediately posterior to the iris stroma is the iris dilator muscle, which is radially oriented and sympathetically innervated. The posterior aspect of the iris is lined with a double layer of densely melanotic epithelial cells (Fig. 6-1). The dorsal aspect of the pupillary margin is capped with a cystic extension of the posterior epithelium, the corpora nigra (or granula iridica) (Fig. 6-2).
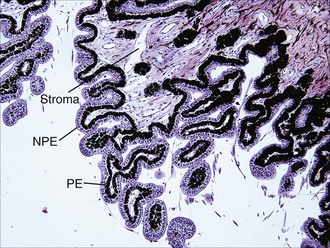
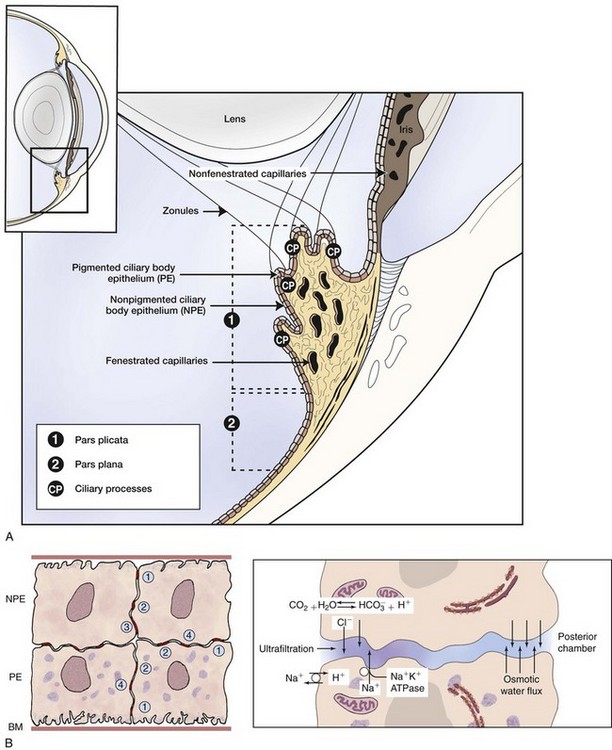
The ciliary body is posterior to the base of the iris and is approximately triangular in cross-section. As viewed from the vitreous cavity, the ciliary body is divided into the anteriorly positioned pars plicata and the posteriorly positioned pars plana. As the name suggests, the pars plicata is characterized by a folded or pleated appearance, although the number, prominence, and shape of the ciliary processes vary among species. In general, species with large anterior chambers, such as horses, have more processes than those with small anterior chambers. Carnivores’ ciliary processes tend to be long and thin, whereas those of herbivores, including the horse, resemble blunted ridges.1 From the ciliary processes, zonular fibers extend and connect to the equatorial region of the lens. The pars plana is a relatively smooth, flat portion that extends from the pars plicata to the most peripheral extension of the retina (the ora ciliaris retinae). The width of the pars plana varies and is widest dorsolaterally.2 The entire inner surface of the ciliary body (the surface in contact with the vitreous body) is lined with a double row of epithelial cells. The innermost epithelial cell layer (from the perspective of the vitreous cavity) is nonpigmented and referred to as the nonpigmented epithelium (NPE) of the ciliary body. The NPE is confluent at the ora ciliaris retinae with the sensory retina, and at the posterior base of the iris with the innermost layer of the posterior iris epithelium. The second epithelial cell layer is heavily melanotic and is referred to as the pigmented epithelium of the ciliary body. The pigmented epithelium lies immediately under the NPE and is contiguous with the retinal epithelium at the ora ciliaris retinae and at the posterior base of the iris with the outermost layer of the posterior iris epithelium. Tight junctions between NPE cells are thought to represent the epithelial portion of the blood-aqueous barrier.3 Deep to the two-layer ciliary body epithelium, each ciliary process has a central portion of connective tissue and a vascular plexus which is fenestrated, allowing leakage of plasma into the ciliary body stroma (Fig. 6-3). The epithelial portion of the blood-aqueous barrier filters this plasma, removing virtually all protein and cells. Thus the aqueous humor represents an ultrafiltrate of plasma. In addition to this filtration function, the NPE contains carbonic anhydrase, which catalyzes the active transport–mediated portion of aqueous humor production (Fig. 6-4).1 Beneath the ciliary processes, lying on the internal surface of the sclera and forming the base of the ciliary body triangle, is the ciliary body musculature. Virtually all mammals have circumferentially oriented, parasympathetically innervated, smooth ciliary body muscles. When these muscles are contracted, the zonular fibers are relaxed, which in turn allows the lens to passively thicken. This mechanism is responsible for accommodation in mammals. However, most nonprimate mammals have poorly developed ciliary body musculature and therefore poor accommodative ability.
Anterior to the ciliary body musculature and visible at the juncture of the base of the anterior face of the iris and the limbus is the ciliary cleft. Spanning the opening of this cleft are strands of connective tissue called pectinate ligaments, which extend from the base of the iris to insert on the inner aspect of the limbus. The ciliary cleft is posterior to the pectinate ligaments and filled with a spongelike network of connective tissue beams, the trabecular meshwork. In the horse, these beams are completely lined with endothelial cells called trabecular cells.1 The ciliary cleft is the location of aqueous humor outflow. Any process that interferes with normal outflow can lead to increased intraocular pressure (see the discussion of equine glaucoma in Chapter 9).
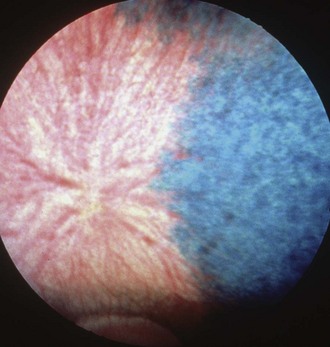
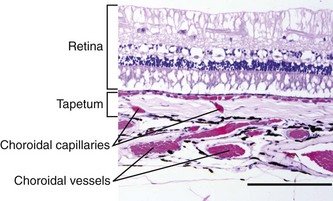
The choroid is the posterior component of the uveal layer and has two main components. Covering the inner surface of the sclera is a thick plexus of vessels embedded in densely melanotic connective tissue that radiate out from the area of the optic disc. Most of these vessels are veins that unite to form the vortex veins near the equator. View of the choroidal vessels is usually obscured ventrally by melanin in the retinal epithelium and dorsally by the tapetum, but may be seen in lightly pigmented individuals (Fig. 6-5).4–6 The tapetum occupies roughly the dorsal half of the fundus and is located immediately interior to the choroidal vasculature. The tapetum varies in color but is usually blue-green to yellow, and is often associated with iris and coat color. While cellular in carnivores, the tapetum in herbivores is made up of highly organized collagenous fibers. In the tapetal area, capillaries from the choroidal arteries run through the tapetal fibers to provide nutrition to the retina. When viewed end-on ophthalmoscopically, these vessels appear as multiple small dots throughout the tapetal area and are called the stars of Winslow (Fig. 6-6). Because the horse has a paurangiotic retinal vascular pattern, the choroid is responsible for supplying nutrition to the entire retina, with the exception of the peripapillar area.
Impact of Uveal Disease on the Equine Industry
Complications associated with equine recurrent uveitis are the number-one cause of blindness in horses worldwide, with annual costs in the United States estimated up to $1 billion.7 The conditions covered in this chapter occur infrequently and sporadically and have little impact on the equine industry from a strictly financial perspective. The importance of veterinarians correctly understanding and managing these diseases is related to the well-being of the individual patient.
Congenital Diseases
Aniridia
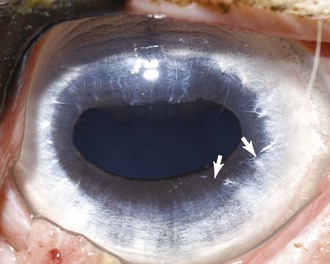
Although the term aniridia implies complete absence of an iris, it has been applied to a clinical condition reported in horses in which only a rudimentary, nonfunctional ridge of iridal tissue is present (Fig. 6-7). Aniridia was first reported in 1955 in a Belgian Draft stallion and 65 of his offspring who were similarly affected.8 The author concluded that aniridia was a heritable defect passed via an autosomal-dominant mode. Subsequently, aniridia was described in a Quarter Horse stallion.9 In an ensuing study, eight offspring of that stallion were followed, and seven demonstrated aniridia.10 The authors concluded the mode of inheritance was similar to that of the Belgian draft stallion. Since then, aniridia has been observed sporadically, being specifically reported in a Thoroughbred colt11 and a Welsh/Thoroughbred cross filly.12
Horses affected with aniridia are usually presented within the first few months of age because the client has noticed such signs as an unusual appearance of both eyes, excessive squinting in bright sunlight, and/or overreacting to flashes of light. On ophthalmic examination, the pupils are widely dilated and nonresponsive. The extent of the pupillary dilation is so marked that the lens equator and ciliary body processes are visible. The corpora nigra is absent. Virtually every reported case of aniridia has been accompanied by what is described as corneal vascularization and nodular changes along the dorsal and sometimes the ventral aspect of the limbus.9–12 When affected globes have been available for histopathologic examination, fine hairs have been found growing from the corneal nodules. These have been identified as dermoids. Cataractous changes are also a frequent accompanying sign with aniridia. The cataracts are usually focal and found in the anterior cortex. They are sometimes present at the time of initial examination but may not arise until later and tend to be slowly progressive. Persistent pupillary membrane (PPM) was reported in one patient.12 Findings on posterior segment examinations in horses with aniridia have been uniformly unremarkable.
The diagnosis of aniridia is straightforward and based on clinical signs alone (see Fig. 6-7). No treatment is available. Many horses with aniridia have performed well in racing or other activities.11,12
Anterior Segment Dysgenesis/Persistent Pupillary Membrane
The term anterior segment dysgenesis is used to describe several syndromes with different clinical presentations. Ophthalmic structures potentially affected by anterior segment dysgenesis include the cornea, iridocorneal angle, iris, ciliary body, lens, and retina, as well as the globe itself as manifested by microphthalmos or buphthalmos.13–16 The severity of signs associated with anterior segment dysgenesis varies greatly. Because most of the structures of the anterior segment are derived from the neural crest, alterations in the differentiation and migration of this layer lead to the signs associated with anterior segment dysgenesis.15 Specific reported malformations involving the anterior uvea include miosis, iris hypoplasia, iris and ciliary body cysts, and persistent pupillary membranes (PPMs). Of these, the most common clinical manifestation of anterior segment dysgenesis in domestic species, including the horse, is PPMs.15 During embryonic development, a vascular plexus called the pupillary membrane covers the anterior surface of the iris and the pupillary aperture. This membrane rarifies near the end of gestation, but remnants can often be found in neonatal foals.16,17 When such strands persist into adulthood, they are called PPMs. In adult horses, it is not uncommon to find small PPMs that originate from the collarette region of the iris and extend a few millimeters. Usually these strands terminate either as a free end in the aqueous or attach to the anterior surface of the iris (Fig. 6-8). Occasionally, PPMs attach to the anterior lens capsule or posterior surface of the cornea. In such instances, opacification is usually seen where the PPMs attach. Such opacities would be present from birth. However, in most equine patients, the distal ends of the PPMs are free floating or attached to adjacent iris. In such instances, no secondary signs are found. In a recent study, the histopathologic changes of apparent PPMs extending from the iris to the posterior surface of the cornea in a 9-week-old Springer spaniel puppy were examined.18 The area of attachment to the posterior cornea was characterized by disruption of Descemet’s membrane and the corneal endothelium. These findings are identical to a developmental disease in humans referred to as Peters anomaly. The authors suggested that this condition should be differentiated from PPMs, which terminate by attaching to adjacent iris or as free floating in the aqueous.
Anterior Uveal Cysts
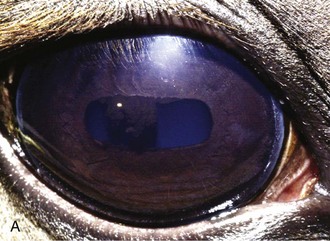
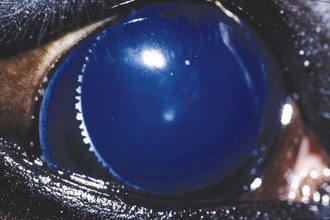
The corpora nigra normally protrudes from the dorsal aspect and to a lesser degree the ventral aspect of the pupillary margin. It is a vacuolated extension of the posterior iris epithelium. Because many horses that have cystic corpora nigra are middle aged or older, it is believed that the condition is not congenital. The cause (or causes) of cystic corpora nigra is not known, but it does not seem to be related to past or present intraocular inflammation.19
When the corpora nigra becomes cystic, its normally roughened appearance becomes smooth and spherical (Fig. 6-9). The condition may be unilateral or bilateral, and the size of the cysts can vary markedly. Most are sufficiently small that they do not cause significant interference with vision. However, depending on the specific location, even moderate-sized cysts can partially inhibit the visual field, especially when the horse is in bright light and the pupil is miotic. For this reason, horses with cystic corpora nigra should be initially evaluated without pharmacologic dilation and under conditions of bright illumination so the extent of pupillary aperture blockage can be accurately assessed.
Although treatment is not ordinarily necessary, the most effective and noninvasive treatment is deflation of the cystic corpora nigra with a laser. In a series of eight horses with signs including loss of vision, being startled when approached from the affected side, head shaking, and poor jumping performance, treatment with an ophthalmic neodymium-yttrium aluminum garnet (Nd:YAG) laser was effective in resolving the vision problems in all horses.19 This procedure does not require general anesthesia; only sedation and an auriculopalpebral nerve block are needed. After surgery, some horses required a short course of topical antiinflammatory and mydriatic medication, but most of the horses were comfortable without treatment. An 810-nm diode laser delivered through an indirect ophthalmoscope headset has also been used successfully to shrink cysts in dogs, cats, and horses.20
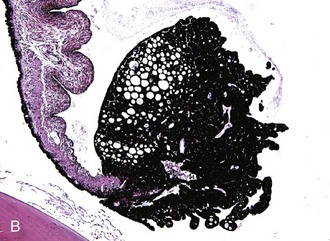
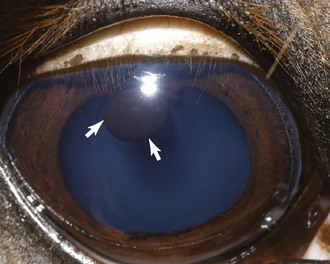
Iridal cysts may be found distinctly separate from the corpora nigra along the margin of the pupil or free floating in the anterior chamber. These cysts are believed to develop in a manner similar to iridal cysts in dogs and represent a failure of the two layers of neuroectoderm to completely fuse, thus allowing fluid to accumulate in areas between the two-layered posterior iris epithelium. Because iridal cysts can enlarge, it has been theorized that some of the posterior iridal epithelial cells that compose the lining of the cyst retain secretory ability.21
The clinical appearance of these cysts is spherical to oval and smooth. Although they can occasionally be transilluminated, most appear opaque (Fig. 6-10). Sometimes the cysts will spontaneously rupture and leave a circular area of pigmentation on the anterior lens capsule or the posterior surface of the cornea.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree