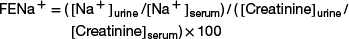Chapter 12 Diseases of the Urinary System
Initial Evaluation of the Urinary Tract
Physical Examination
The penis should be exteriorized and the prepuce and free portion of the penis examined. This part of the evaluation can be accomplished in an unsedated animal by either “sitting” it on its rump or placing it in lateral recumbency with the upper hindlimb pulled forward (see Chapters 1 and 8). Examination of distressed animals may require use of local anesthesia or sedation. Sedation may be achieved by use of acepromazine (0.05 to 0.1 mg/kg given intravenously [IV] or intramuscularly [IM]) or diazepam (0.1 mg/kg by slow intravenous infusion); alternatively, a lumbosacral epidural block with 2% lidocaine (1 mL/7 kg) may be used instead of sedation to relieve discomfort and aid in exteriorization of the penis. The use of xylazine should be avoided in animals with potential obstruction owing to its diuretic effects,1,2 which are associated with increased risk of urinary tract rupture if obstruction is present.
Ancillary Diagnostic Testing
Complete Blood Count and Serum Biochemistry Panel
The results of a complete blood count (CBC) and a serum chemistry panel can provide assistance in the diagnosis, prognosis, management, and monitoring of diseases involving the urinary tract. Values for blood analysis in animals with urinary tract disease, however, may be within normal limits (as established in reference ranges), depending on disease severity and duration. For this reason, this section focuses on interpretation of abnormalities once they are identified (see Appendix 2, Tables 2-1 to 2-3).
Abnormalities noted on the CBC may include anemia of chronic inflammation or renal failure, a stress or inflammatory leukogram, and hyperfibrinogenemia. Anemia of chronic inflammation is a nonregenerative anemia characterized by normocytic, normochromic red blood cells, and the anemia typically is mild to moderate in severity.3 The pathomechanisms of this anemia include increased concentrations of inflammatory mediators, which reduce red blood cell lifespan and impair bone marrow function. Anemia of chronic renal failure is also normocytic and normochromic, but the anemia may become more severe than anemia of chronic inflammation. The mechanism for this anemia is decreased renal production of erythropoietin in the kidneys.3 The long red blood cell lifespan in ruminants (125 to 160 days)4 precludes the development of anemia in acute renal failure (ARF).
Inflammatory diseases are common in sheep and goats, and the leukogram may reflect inflammatory processes primarily affecting the upper urinary tract. Most ruminants have a neutrophil-lymphocyte (N:L) ratio of 1:2, although in adult goats the ratio typically is 1:1. The N:L ratio is a more important consideration than are the actual numbers of each cell type. Sheep and goats have a small circulating pool of neutrophils, so neutropenia typically develops at 24 to 48 hours after the onset of severe inflammation, with a consequent reduction in the N:L ratio. The presence of immature neutrophils (bands) is termed a “left shift” and indicates severe inflammation. This is a common finding in the acute phase of severe inflammation but is associated with a poor prognosis if the left shift persists. Increases in the N:L ratio with the presence of bands indicates an inflammatory leukogram. A reversal of the N:L ratio to greater than 2:1, but without the presence of bands, is indicative of a stress leukogram. The stress leukogram occurs as a result of corticosteroid administration or endogenous steroid release, generally from noninflammatory diseases. Fibrinogen is a positive acute-phase protein, increasing over a period of 2 days after initiation of inflammation in ruminants.5,6
Normal sheep have an FE of sodium of less than 1%,7 whereas an FE of sodium greater than 1% indicates primary renal tubular disease or sodium toxicity. Postrenal azotemia is most commonly caused by urinary tract obstruction, which is identified by findings in the history, physical examination, and imaging studies. With prolonged urinary tract obstruction, renal damage may occur, worsening the azotemia.
Hyponatremia and hypochloremia may be present with renal disease, both as consequences of renal losses and decreased dietary intake. Hyperkalemia, as is seen in monogastrics with renal failure, is not consistently seen in ruminants with urinary obstruction or renal disease. Protection from this disturbance may be afforded by aldosterone release in response to hypovolemia, thereby preserving sodium.8 This in turn allows potassium to replace sodium as the major cation in the saliva, resulting in sequestration in the gastrointestinal tract. Animals with metabolic acidosis may also show hyperkalemia as potassium is shifted extracellularly. Phosphorus is primarily excreted by ruminants into the saliva, not by the kidney as in other species.9 In lambs, only 3% of total phosphorus excretion occurs through the kidney.9 Therefore conditions that cause a reduced GFR do not necessarily result in increased serum phosphorus. When phosphorus is elevated, however, it should be considered significant. Mild hypocalcemia also may be noted, particularly in hyperphosphatemic animals as a result of complexing of these two ions. Hypermagnesemia is also associated with decreased GFR. The acid-base status of animals with urinary tract disease is variable and can be partially evaluated by determination of mean total CO2 (TCO2) on serum chemistry studies, with a high TCO2 indicating metabolic alkalosis and a low value indicating metabolic acidosis (see Appendix Table 2-4).
Urinalysis
Urinalysis should be performed in any animal with suspected urinary tract disease or any other systemic disease for which the disease or treatment may impact urinary health. Free-catch urine may be obtained spontaneously during physical examination, or animals may be encouraged to urinate by occlusion of the nostrils (sheep), placement in a new clean stall, exposure to a new animal, or allowing the animal to lie down for a time and then getting it up. Animals that do not voluntarily provide a urine sample and have a patent urinary tract may be catheterized, or cystocentesis may be performed. Rams, bucks, and castrated males also possess a urethral diverticulum or recess, at the level of the ischial arch, that communicates with the urethra and contains the ducts of the bulbourethral glands.10 This structure readily accepts a urinary catheter, preventing retrograde catheterization of the urinary bladder. Catheterization of males is possible through the use of J-curved human cardiac catheters.11 In ewes and does, a suburethral diverticulum is present below the external urethral orifice, which must be bypassed to allow retrograde catheterization of the urinary bladder.
After an adequate specimen has been obtained, the urine is subjected to gross examination for color and clarity. Commercial dipsticks are available for biochemical testing. In addition, a handheld refractometer can be used for specific gravity determination, with centrifugation at 450g for 3 to 5 minutes, followed by examination of the sediment and supernatant.12
Urine specific gravity is useful for investigating the origin of azotemia and should be determined with a refractometer, rather than by urine dipstick testing, which carries an upper limit of 1.025 to 1.030.11 Urine-concentrating ability is lost before the occurrence of azotemia, so the production of dilute urine in azotemic animals suggests loss of renal function, with a specific gravity greater than 1.025 considered to reflect adequate concentrating ability in ruminants. Urine specific gravity should be interpreted carefully and not based on a single sample, as indicated by personal observation (by M.J.) of values from as low as 1.003 in clinically normal goats without added dietary salts.
Urine dipstick testing is now available for biochemical determinations of urine pH, protein, glucose, ketones, occult blood, bilirubin, urobilinogen, nitrites, and urine specific gravity. Urine pH is best determined on a pH meter,13 but urine dipstick measurement can provide a useful indication. In ruminants, the pH normally is alkaline, with urine pH generally between 7.5 and 8.5.14 Sheep and goats commonly experience a paradoxical aciduria in the presence of metabolic alkalosis associated with abomasal or proximal intestinal obstruction,15 but this also can occur with the significant metabolic and acid-base derangements characteristic of severe urinary tract disease. Any of a variety of pathophysiologic mechanisms related to volume, sodium, chloride, and potassium depletion may be responsible for the aciduria.15
Urine normally contains very low quantities of protein, and urine dipstick analysis normally shows no or only trace amounts. However, the normal alkaline urine of sheep and goats influences the protein reaction, leading to falsely elevated protein readings14 of 1+ or 2+. To definitively determine if elevated protein levels exist, the sulfosalicylic acid turbidity test or colorimetric assays should be performed. If proteinuria is determined to be present, postrenal contributions should be considered when urine was obtained as a free-catch specimen. These include cystitis, urethritis, and other exudative processes of the distal urinary tract. Proximal causes of proteinuria include prerenal (e.g., hemoglobin from intravascular hemolysis and myoglobin) and postrenal (e.g., inflammatory or degenerative glomerular or tubular damage) causes.14 Glomerular protein losses tend to be of greater magnitude and result in significant reductions in blood protein levels. Proteinuria may be present in neonatal lambs and kids until approximately 2 days of age as a result of renal permeability to colostral proteins.16
Normally, the urine glucose reaction should be negative. The renal threshold for glucose in ruminants is considered to be 100 to 140 mg/dL,17 although one study reported a renal glucose threshold in goats as low as 81 mg/dL.18 Blood glucose levels above this threshold range will result in glucosuria, with common causes including Clostridium perfringens type D enterotoxemia19 and corticosteroid, xylazine,1 or dextrose administration. Less common causes include stress and renal tubular disease.14
Urine ketone concentrations are useful for detecting excessive fat metabolism, as seen with negative energy balance syndromes, including pregnancy toxemia and starvation (see Chapters 2 and 8). Determination of the urine ketone concentration is the single most useful test for diagnosis of pregnancy toxemia in ewes and does (Figure 12-1). Of the three types of ketone bodies produced by the body, urine ketone strips detect acetoacetate and acetone but not beta hydroxybutyrate, the primary ketone produced.14 False-negative or underestimated ketone concentrations may therefore be obtained as a consequence of the volatility of ketone bodies if sample testing is delayed or if beta hydroxybutyrate does not account for a large proportion of the ketone bodies produced in an individual animal.
A positive test for urine occult blood can indicate the presence of hemoglobin, myoglobin, or whole blood in the urine sample. Differentiating these can be performed in a stepwise fashion, particularly if the urine is visibly pigmented. Red or brown color cannot be relied on to indicate the presence of hemoglobin or myoglobin, respectively (Figure 12-2). First, the urine sample should be centrifuged and the sediment examined. If the supernatant loses pigmentation and the sediment is composed primarily of red blood cells, hematuria is present and indicates hemorrhage or an inflammatory condition. If the supernatant remains red or brown and no sediment is produced or or if the sediment does not contain intact red blood cells, hemoglobinuria or myoglobinuria exists. At this time, a blood sample should be drawn, centrifuged in a microhematocrit tube, and observed for evidence of hemolysis, including pink plasma and anemia. If no evidence of hemolysis exists, myoglobinuria is the most likely diagnosis and may be confirmed by clinical examination, history, and elevations of muscle enzymes on a serum chemistry panel. Myoglobin is a much smaller molecule than hemoglobin and passes more readily into the urine. It will be present in the urine without being visible in the plasma. Hemoglobin, however, accumulates in the blood and then, upon exceeding the renal threshold, will be filtered into the urine. Hemoglobin, if visible in the urine, will therefore be visible in the plasma.
Potential causes of hematuria, hemoglobinuria, and myoglobinuria are summarized in Box 12-1. Diseases that cause purely extravascular or spleen-mediated hemolysis (e.g., anaplasmosis) will not result in hemoglobinuria, which is produced only when intravascular hemolysis exists. Hypophosphatemic hemoglobinuria has been rarely reported in sheep and goats with a feeding history that includes Brassica species.20,21 Neonatal isoerythrolysis has been reported in lambs and kids fed cow colostrum,22 but hemoglobinuria does not appear to be a common clinical finding. Cold water isoerythrolysis has been reported in a variety of species after rapid consumption of large amounts of cold water. The condition occurs as a result of fragility of red blood cells from the reduction in plasma osmolality. The red blood cells of goats exhibit increased osmotic fragility, making this species the most sensitive to the condition.23
Bilirubinuria (conjugated bilirubin) may be present as a result of hemolytic disease, hepatic insufficiency, or biliary obstruction. Of note, urobilinogen, nitrites, and urine specific gravity, as determined by dipstick testing, are not considered diagnostic in veterinary medicine.12,14
Urinary gamma-glutamyltransferase (GGT) concentration, available through reference laboratories, has been shown to be of diagnostic value in nephropathies in sheep and goats owing to presence of the enzyme in proximal tubular cells, where serum concentrations will not be affected.24,25 In two different studies, urine GGT concentrations in normal adult sheep were reported at 5 to 33 U/L (mean, 13.9 U/L)7 and 6.8 to 24.6 U/L (mean, 15.7 U/L)27 respectively. Urinary GGT levels have been shown to increase a mean of 4.5 days after experimental aminoglycoside-induced nephrotoxicosis in sheep.27
Urine sediment examination is performed to identify the presence of cells, bacteria, casts, crystals, or other debris. Cells, typically erythrocytes, leukocytes, and epithelial cells, may originate from any level of the urinary tract. Red and white blood cells degenerate quickly in urine and can only be accurately identified in fresh samples. Large amounts of erythrocytes indicate the presence of hematuria as a cause of red urine or a positive fecal occult blood test; considerations in the differential diagnosis for red or brown urine are listed in Box 12-1. The presence of large numbers of leukocytes, particularly neutrophils, indicates the presence of inflammatory exudates, which most commonly originate from the renal pelvis or urinary bladder. If bacteria are noted on urinalysis, it is important to determine if they are contaminants or the cause of urinary tract inflammation. The presence of white blood cells, in addition to bacteria, suggests legitimate bacterial presence. A cystocentesis sample, along with bacterial culture, should be obtained to further clarify this. When accompanied by dysuria or stranguria, this exudate probably originates from the lower urinary tract, whereas accompanying signs of systemic illness would indicate an origin in the upper urinary tract. Considerations in the differential diagnosis for pyuria include contamination of the prepuce or female reproductive tract, pyelonephritis, cystitis, urolithiasis, and neoplasia. Epithelial cells normally are present in low numbers in the urine, so large numbers generally indicate contamination at collection, but in such cases the cells should be confirmed to be non-neoplastic.14
BOX 12-1 Differential Diagnosis for Red- or Brown-Pigmented Urine
Contamination from reproductive tract
Disseminated intravascular coagulation
Water intoxication/isoerythrolysis (most common in goats)
Bacillary hemoglobinuria (Clostridium haemolyticum)
Plant toxicity: Brassica, onion
Severe myodegeneration/myositis
Urinary casts are forms of proteins or cells that originate in the kidney. Hyaline casts are protein-only casts and indicate glomerular protein leakage, and the formation of these casts is increased with highly concentrated or acidic urine. Cellular casts may be made up of red or white blood cells or epithelial cells and indicate hemorrhage, infection, or tubular sloughing, respectively, all of renal origin. Granular casts and waxy casts are casts that originally were cellular but have been degraded. Casts may be broken down in alkaline urine; findings should therefore be interpreted only for specimens of freshly obtained urine.14
Crystalluria is important in small ruminant urinalysis, owing to the commonality of urolithiasis in small ruminants. The most common urolith components include struvite (magnesium ammonium phosphate), apatite (calcium phosphate), calcium carbonate, and silicate. Crystals may be present in clinically normal animals owing to the alkaline urine of ruminants and dietary contributors and should be interpreted in light of other risk factors for urolithiasis to determine case management. Conversely, as encountered in our own experience, the urine of obstructed animals obtained from cystocentesis or cystotomy often is free of crystals (see Appendix 2, Table 2-7).
Ultrasound Examination
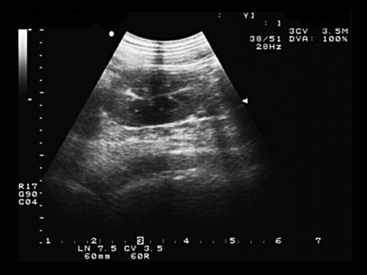
Transabdominal ultrasound imaging is more frequently used than transrectal examination for urinary tract evaluation in small ruminants. The kidneys and urinary bladder are readily evaluated as well as surrounding soft tissue structures, swellings, and the peritoneal cavity. The ureters and urethra may be impossible to identify in normal sheep or goats.28,29 For transabdominal evaluation, a 3.5- or 5-mHz curvilinear or linear probe typically is used, with the left kidney situated in the dorsal region of the right paralumbar fossa and the right kidney visualized dorsally in the 11th and 12th intercostal spaces.30 In sheep and goats, the kidney is smooth, lacking the lobulation seen in bovine kidneys29,30 (see Figures 12-3 and 12-4). Reference ranges for the ultrasonographic evaluation of the urinary tract in sheep have been published.28,29 In ewes weighing between 41 and 89 kg, the mean length, width, and depth of the left kidney were 8.2 cm, 4.4 cm, and 4.0 cm, respectively.29 Rams of the same size range had mean left kidney measurements of 8.4 cm in length, 4.7 cm in width, and 4.4 cm in depth, similar to values obtained for the right kidney.28
Abnormalities frequently noted on renal ultrasound examination include hydronephrosis, pyelonephritis, cysts, neoplasms, and perirenal fluid accumulation. Hydronephrosis is evidenced by a dilated collection system filled with anechoic fluid. Pyelonephritis is marked by renal enlargement with dilated renal sinus, containing echogenic debris in varying amounts.31 The ureters also may be dilated.31 Cysts and neoplastic masses may also be noted as hypoechoic fluid-filled or solid masses on the surface of the kidney or within the renal parenchyma. Perirenal fluid accumulation may be inflammatory in origin but is more commonly secondary to urinary tract rupture, in which the fluid will be anechoic.
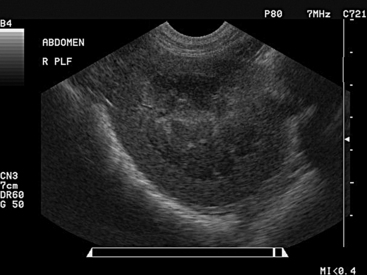
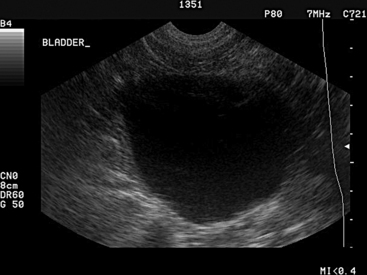
On ultrasound imaging, the urinary bladder is visualized in the right inguinal region or may be examined transrectally. Urinary bladder diameter, wall thickness, mural changes, and intraluminal contents may be evaluated. In female sheep, the diameter of the urinary bladder ranged from 0.3 to 6.9 cm in 96.8% of sheep, with a mean diameter of 3.6 ± 1.6 cm.29 In rams, urinary bladder diameter ranges between 1.8 and 13.2 cm, with a mean of 7.5 ± 2.8 cm. In goats with obstructive urolithiasis, the urinary bladder was distended to 4 to 15 cm (mean 7 cm) and 8 to 12 cm (mean 9.5 cm) in small and large breed goats, respectively,32 so overlap of bladder diameters is observed in goats with patent and those with nonpatent urinary tracts. Wall thickness varies in accordance with the degree of bladder filling, with the wall thickness normally decreasing as bladder volume increases.29 Therefore a thick wall in a distended bladder may indicate inflammation or other mural infiltration. The wall of the urinary bladder also should be examined for the presence of nodules or other abnormalities along the interior or exterior of the urinary bladder. Normal urine within the urinary bladder is anechoic, but a common finding is some minor, echogenic debris within the bladder lumen (Figure 12-5). With hematuria, pyuria, or urinary calculosis, the ventrum of the urinary bladder may contain hyperechoic material. During transabdominal ultrasound examination, with the probe in contact with the abdominal wall, the operator may shake the probe and abdominal wall vigorously to determine and demonstrate the presence of cellular debris, blood clots, or uroliths within the urinary bladder, differentiating this from masses associated with the bladder wall.
Transabdominal ultrasound examination also is useful for determining the presence of excess free abdominal fluid. Visual determination of the character of the fluid on ultrasound imaging is the first step in identification and classification of the fluid type. Anechoic fluid signifies a transudate or modified transudate, as would be seen with urine leakage, whereas fluid with echoic (cells or protein) debris is consistent with inflammatory or exudative processes. For thorough characterization of fluid, abdominocentesis should be performed as described later in this chapter (see also Chapter 5).
Cystocentesis
Needle aspiration of urine directly from the urinary bladder avoids potential contamination of urine by the lower urinary tract, providing superior samples for laboratory evaluation, including bacterial culture, and also may be used in the treatment of obstructive urolithiasis if the urinary bladder is intact.33 With the animal restrained in left lateral recumbency, the urinary bladder should be identified low in the right flank by deep abdominal palpation or transabdominal ultrasonography. The skin surface is clipped and aseptically prepared, and an 18-gauge, 2-3.5 inch needle with syringe attached is inserted perpendicularly through the skin and abdominal wall and quickly thrust into the bladder lumen. The needle is steadied, at least 10 mL of urine is aspirated, and the needle is quickly withdrawn. Quick, sharp insertion and removal of the needle from the bladder will ensure that only a small circular perforation of the urinary bladder wall is made, which will be quickly sealed. Larger, slit-shaped perforations, particularly those made into a distended urinary bladder wall with poor tissue integrity, may result in uroperitoneum or sepsis, although these complications appear to be rare.33
Abdominocentesis
Fluid obtained should be examined for total protein level, cytologic count and differential, and creatinine level if uroperitoneum is suspected. Normal peritoneal fluid from ruminants should be clear and colorless to straw-colored. Normal values for total protein, total nucleated cell count, and differential count vary widely in cattle.34–36 We typically consider peritoneal fluid to be within normal limits if it is not present in large amounts, has a total protein less than 3.0 g/dL and a total nucleated cell count less than 5000 cells/μL. With abnormal exudates, classification should be based on the pathophysiology behind their creation, rather than on protein and cell counts alone.37
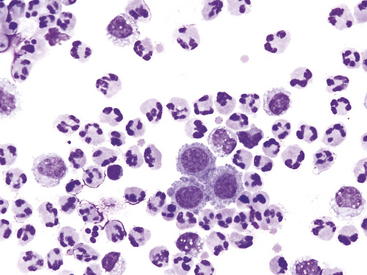
Protein-poor transudates result from excess diffusion of water or lymph from the vascular space as a result of abnormalities of hydraulic or oncotic pressure. They typically have a total protein less than 2.0 g/dL and a total nucleated cell count less than 1500 cells/μL. Causes may include protein-losing enteropathy or nephropathy, lymphatic obstruction, and portal hypertension. Protein-rich transudates result from inflammatory processes, which increase vascular permeability so that plasma exits the vasculature, often along with leukocytes. These generally have a total protein greater than 2 g/dL and a total nucleated cell count greater than 5000 cells/μL and are caused by bacteria, some viruses, protozoa, parasites, neoplasms, foreign bodies, or uroperitoneum. Hemorrhagic effusions must be separated into iatrogenic (occurring at the time of abdominocentesis) and pathologic causes, on the basis of several criteria.38 These effusions have a total protein greater than 2.0 g/dL and a total nucleated cell count greater than 2000 cells/μL and may be caused by trauma, bleeding disorders and neoplasic diseases. Effusions caused by a rupture of a hollow organ or other tissue include those resulting from urinary tract rupture, biliary leakage, and gastrointestinal rupture. In early phases of the disease, uroperitoneum will have the character of urine (very low TP and total nucleated cell count), but with time and irritation to the peritoneum will take on characteristics of an exudates with increased TP and total nucleated cell count, which may be diluted by high volumes of urine leakage. Early uroperitoneum will usually have a total protein less than 2.0 g/dL and a total nucleated cell count less than 1500 cells/μL, but with chronicity will have a variable total protein and total nucleated cell count less than 1500 cells/μL.37,38 Figure 12-6 shows the cytologic findings for peritoneal fluid obtained from an animal with uroperitoneum of a few days’ duration. Additional but less common effusions include lymphorrhage from lymphatic leakage and multiple-process effusions, in which multiple pathophysiologic processes alter the character of the peritoneal fluid.37
The most common biochemical test performed on peritoneal fluid samples from ruminants is a creatinine concentration determination. This test can definitively detect the presence of urine in the abdomen, with creatinine levels greater than twice the serum creatinine concentration indicating that uroperitoneum exists.39
Radiography
Survey Radiography
The number, size, shape, and density of the kidneys are evaluated. Unilateral and bilateral renal agenesis has been reported in lambs.40,41 In goats, normal kidneys have been found to be 2 to 2.5 times the length of the second lumbar vertebra.42 Enlarged kidneys may be seen with hydronephrosis, amyloidosis, glomerulonephritis, cysts, or compensatory hypertrophy of a functional kidney. With these conditions, kidneys also may be of normal size early in the disease process. Small kidneys are typical findings in end-stage chronic renal disease. Air or mineral opacities may be present in the kidneys, suggestive of abscesses or trauma (air) and uroliths (mineral). The ureters are best visualized with contrast radiography, but air or mineral opacities may be present within the lumen.
The urethra of rams, bucks, and castrated males is an important structure to be evaluated in imaging studies. Survey radiographs are most useful to evaluate the tissues surrounding the urethra and opacities within the urethra. In one study,32 cystic or urethral calculi were visible in 8 of 10 obstructed goats. The visible stones seen in these studies were calcium carbonate or struvite in composition, while apatite and silicate stones were not seen radiographically.32 In other studies,11,43 survey radiographs were of limited usefulness for diagnosing uroliths. Negative findings on survey radiographs should not be interpreted to rule out urolithiasis. Evaluation of the pelvic bones also is important owing to the possibility of impairment of urethral and bladder patency secondary to fracture. The urethra is best studied by contrast radiography or endoscopy. Endoscopy is not frequently used in small ruminant urethral studies owing to the requirements for a smaller-diameter, shorter endoscopy unit.
Contrast Radiography
An excretory urogram provides an anatomic and qualitative functional view of the kidneys. The procedure involves the intravenous injection of an ionic contrast medium, with sequential radiographs taken up to 40 minutes after injection. Patients undergoing excretory urography should be adequately hydrated, because opacification of the kidneys is dependent on glomerular filtration. In normal goats, the kidneys are best visualized if radiographs are taken immediately after injection.42 Delayed or reduced opacification indicates dehydration or inadequate glomerular filtration. In evaluation of ureteral patency, an important consideration is that normal peristalsis can appear as a stricture or narrowing of the lumen. Ureteral patency can be altered by uroliths, blood clots, inflammatory exudates, trauma, or stricture. Excretory urography, in some patients, may not provide visualization of the urinary bladder and urethra.43
Contrast cystography and urethrography may be performed in normograde or retrograde fashion and with negative (air) or positive (organic iodide) contrast media. Barium should never be used for urinary imaging. Indications for such procedures include dysuria, pollakiuria, and chronic hematuria. Cystourethrography is best performed in a normograde fashion through a cystotomy tube.43 For retrograde studies, catheterization may be performed completely only in the female ruminant but may be partially completed in male ruminants. Occlusion of the distal urethral orifice after partial catheter passage allows for the instillation of contrast media in retrograde fashion. Alternatively, a precurved cardiac catheter may be utilized to bypass the urethral diverticulum.11 For retrograde studies, a ballooned catheter is used and passed into the urinary bladder in females, or a nonballooned catheter is passed a few inches into the bladder in males, and contrast material is instilled. Use of intraluminal contrast material allows for assessment of degree of patency and wall thickness of the urinary bladder and urethra. Mural masses may be visualized and may be caused by cellular or fibrous infiltration. Filling defects of the urinary bladder may be caused by polyps, air, calculi, blood clots, or inflammatory exudates, and urachal diverticula may be seen.44 Filling defects of the urethra may be caused by air bubbles, calculi (which may be radiopaque or radiolucent), blood clots, neoplasms, inflammation, scar tissue, or extramural compression. Extravasation of contrast material may result from traumatic lacerations, fistulas (urethrorectal and urethrovaginal), and diverticula. Fistula between the urethra and corpus spongiosum has been diagnosed by contrast radiography in a goat-sheep after surgery for obstructive urolithiasis.44
Renal Biopsy
The biopsy procedure should be performed in a well-restrained or adequately sedated animal under ultrasound guidance. The skin overlying the last 2 or 3 ribs and paralumbar region is aseptically prepared, the skin and body wall are anesthetized with 2% lidocaine, and a stab incision is made for introduction of the biopsy instrument. The ultrasound probe may be placed in a sterile glove filled with ultrasound gel to maintain asepsis and to locate the target kidney. A 14-gauge biopsy instrument is directed into the kidney parenchyma and a sample obtained. Depending on the testing required, the biopsy specimen should be divided, with both fresh and fixed tissue submitted to a reference laboratory. Potential complications of renal biopsy include hematuria, hematoma, hemoabdomen, and peritonitis. In a retrospective study in 25 cattle, ultrasound-guided percutaneous renal biopsy resulted in a small subcapsular hematoma (less than 2 cm in diameter) after the procedure in 6 of the animals, but no gross or occult hematuria.45 Another study using serial laparoscopic biopsies in cattle resulted in microscopic hematuria for 1 to 5 days.46
1. DeRossi R., Juniqueira A.L., Beretta M.P. Analgesic and systemic effects of ketamine, xylazine, and lidocaine after subarachnoid administration in goats. Am J Vet Res. 2003;64:51-56.
2. Thurmon J.C., et al. Effects of xylazine hydrochloride on urine in cattle. Aust Vet J. 1978;54:178-180.
3. Waner T., Harrus S. Anemia of inflammatory disease. In: Feldman B.F., Zinkl J.G., Jain N.C., editors. Schalm’s Veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:205-209.
4. Kramer J.W. Normal hematology of cattle, sheep, and goats. In: Feldman B.F., Zinkl J.G., Jain N.C., editors. Schalm’s Veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:1075-1084.
5. Braun U., Stehle C., Ehrensperger F. Clinical findings and treatment of listeriosis in 67 sheep and goats. Vet Rec. 2002;150:38-42.
6. de la Concha-Bermejillo A., et al. Severe persistent orf in young goats. J Vet Diagn Invest. 2003;15:423-431.
7. Garry F., et al. Renal excretion of creatinine, electrolytes, protein, and enzymes in healthy sheep. Am J Vet Res. 1990;51:414-419.
8. Mitchell A.R., Moss P. Responses to reduced water intake, including dehydration natriuresis, in sheep excreting sodium predominantly in urine or in faeces. Exp Physiol. 1995;80:265-274.
9. Ammerman C.B., et al. Ruminant utilization of inorganic phosphates. J Anim Sci. 1957;16:796-810.
10. Garrett P.D. Urethral recess in male goats, sheep, cattle, and swine. J Am Vet Med Assoc. 1987;191:689-691.
11. Van Weeren P.R., Klein W.R., Voorhout G. Urolithiasis in small ruminants II. Cysto-urethrography as a new aid in diagnosis. Vet Q. 1987;9:79-82.
12. Osborne C.A., Stevens J.B. Urinalysis: a clinical guide to compassionate patient care. Shawnee Mission, Kan: Bayer Corporation; 1999.
13. Nappert G., Naylor J.M. A comparison of pH determination methods in food animal practice. Can Vet J. 2001;42:364-367.
14. Stockham S.L., Scott M.A. Urinary system. In: Stockham S.L., Scott M.A., editors. Fundamentals of veterinary clinical pathology. ed 2. Ames, Iowa: Blackwell; 2008:277-336.
15. Lunn D.P., et al. Renal net acid and electrolyte excretion in an experimental model of hypochloremic metabolic alkalosis in sheep. Am J Vet Res. 1990;51:1723-1731.
16. McDougall EI: Proteinuria of newborn suckling ruminants, Biochem J 94:101–105, 1095.
17. Carlson G.P. Clinical chemistry tests. In: Smith B.P., editor. Large animal internal medicine. ed 3. St Louis: Mosby; 2002:389-412.
18. Cutler J.T. Studies of the carbohydrate metabolism of the goat. J Biol Chem. 1934;106:653-666.
19. Uzal F.A., Kelly W.R. Experimental Clostridium perfringens Type D enterotoxemia in goats. Vet Pathol. 1998;35:132-140.
20. Setty D.R.L., Narayana K. A case of non-febrile haemoglobinuria in a she goat. Indian Vet J. 1975;52:149.
21. Stamp J.T., Stewart J. Haemolytic anaemia with jaundice in sheep. J Comp Pathol. 1953;63:48-52.
22. Winter A., Clarkson M. Anaemia in lambs and kids caused by feeding cow colostrum. In Pract. 1992;14:283-286.
23. Perk K., Frie Y.F., Herz A. Osmotic fragility of red blood cells in young and mature domestic and laboratory animals. Am J Vet Res. 1964;25:1241-1248.
24. Fernandez A., et al. Clinicopathological features in ovine AA amyloidosis. Res Vet Sci. 2003;75:203-208.
25. Price R.G. Urinary enzymes, nephrotoxicity and renal disease. Toxicology. 1982;23:99-134.
26. Garry F., et al. Renal excretion of creatinine, electrolytes, protein, and enzymes in healthy sheep. Am J Vet Res. 1990;51:414-419.
27. Garry F., Chew D.J., Hoffsis G.F. Enzymuria as an index of renal damage in sheep with induced aminoglycoside nephrotoxicosis. Am J Vet Res. 1990;51:428-432.
28. Braun U., Schefer U., Fohn J. Urinary tract ultrasonography in normal rams and in rams with obstructive urolithiasis. Can Vet J. 1992;33:654-659.
29. Braun U., Schefer U., Gerber D. Ultrasonography of the urinary tract of female sheep. Am J Vet Res. 1992;53:1734-1739.
30. Hallowell GD: Abdominal ultrasound. Proceedings of the 26th Annual American College of Veterinary Internal Medicine Forum, San Antonio, Tex, 2008.
31. Floeck M. Sonographic application in the diagnosis of pyelonephritis in cattle. Vet Radiol Ultrasound. 2007;48:74-77.
32. Halland S.K., House J.K., George L.W. Urethroscopy and laser lithrotripsy for the diagnosis and treatment of obstructive urolithiasis in goats and pot-bellied pigs. J Am Vet Med Assoc. 2002;12:1831-1834.
33. Janke J.J., et al. Use of Walpole’s solution for treatment of goats with urolithiasis: 25 cases (2001-2006). J Am Vet Med Assoc. 2009;234:249-252.
34. Kopcha M., Schulze A.E. Peritoneal fluid. II. Abdominocentesis in cattle and interpretation of noneoplastic samples. Comp Cont Educ Pract Vet. 1991;13:703-710.
35. Anderson D.E., et al. Comparative analyses of peritoneal fluid from calves and adult cattle. Am J Vet Res. 1995;56:973-976.
36. Wilson A.D., Hirsch V.M., Osborne A.D. Abdominocentesis in cattle: technique and criteria for diagnosis of peritonitis. Can Vet J. 1985;26:74-80.
37. Stockham S.L., Scott M.A. Cavity effusions. In: Stockham S.L., Scott M.A., editors. Fundamentals of veterinary clinical pathology. ed 2. Ames, Iowa: Blackwell; 2008:831-868.
38. Wilson D.G., MacWilliams P.S. An evaluation of the clinical pathologic findings in experimentally induced urinary bladder rupture in pre-ruminant calves. Can J Vet Res. 1998;62:140-143.
39. Sockett D.C., et al. Metabolic changes due to experimentally induced rupture of the bovine urinary bladder. Cornell Vet. 1986;76:198-212.
40. Dennis S.M. Urogenital defects in sheep. Vet Rec. 1979;105:344-347.
41. Hartley W.J., Kater J.C. Perinatal disease conditions of sheep in New Zealand. N Z Vet J. 1964;12:49-57.
42. Cegarra IJ, Lewis RE: Excretory urography in the goat (Capra hircus), Am J Vet Res 38:1129–113, 19772.
43. Palmer J.L., et al. Contrast radiography of the lower urinary tract in the management of obstructive urolithiasis in small ruminants and swine. Vet Radiol Ultrasound. 1998;39:175-180.
44. Cruz-Arambulo R de J, et al. What is your diagnosis? Communication between the urethra and the corpus spongiosum, urethral stricture, mild cystitis, and presence of a urachal diverticulum. J Am Vet Med Assoc. 2003;222:1211-1212.
45. Mohamed T., Oikawa S. Efficacy and safety of ultrasound-guided percutaneous biopsy of the right kidney in cattle. J Vet Med Sci. 2008;70:175-179.
46. Naoi M., et al. Laparoscopic-assisted serial biopsy of the bovine kidney. Am J Vet Res. 1985;46:699-702.
Diseases of the Kidneys
In clinical practice, kidney disease is not commonly encountered as a primary problem in small ruminants; however, incidental kidney pathology often can be identified at necropsy.1 Kidney disease is described based on duration (acute versus chronic) and the character of renal damage leading to dysfunction (glomerular, tubular, and vascular). The clinician’s challenge is to recognize situations resulting from primary renal disease or risks leading to secondary or induced renal damage. Clinical tendencies with kidney disease are anuria, oliguria, dysuria, abdominal pain, and abnormal urinary constituents. Appearance of clinical signs in small ruminants often is synchronized with the multisystem disease processes that lead to renal damage. Therefore recognition of risk factors, preemptive case management practices, ancillary diagnostics, and postmortem diagnosis are important in overall disease management and prevention.
General causes of kidney disease are:
• Infectious (bacterial, viral, and parasitic)
• Toxic (chemical, heavy metals, medications, and plant origin)
• Obstruction and trauma (nephroliths, direct)
• Secondary hydronephrosis from ureteral, cystic, and urethral calculi
• Vascular (infarcts, hyperdynamics of sepsis and toxemia)
• Chronic inflammation (glomerulonephritis, amyloidosis)
Renal Failure
Renal failure occurs when diminished renal function results in persistent metabolic abnormalities such as azotemia as well as the inability to concentrate urine. Renal failure that develops rapidly, within a few hours or days, constitutes acute renal failure (ARF) and usually is due to intrinsic (vascular, toxic) causes from systemically absorbed toxins, body origin toxins (myoglobin, hemoglobin, urea), administered therapeutics, or dynamic changes in renal blood flow with sepsis, shock, or toxemia. The kidneys receive a large proportion of the circulating blood volume, resulting in high rates of toxin exposure, as well as increased vulnerability to ischemia and reperfusion injury with diseases causing hyperdynamic changes in nutrient blood flow. Toxin exposure is amplified as renal tubules resorb filtered toxins in conjunction with the normal function of urine concentration. Damage to this sensitive portion of the nephron may result in acute tubular necrosis, eventuating in loss of urine-concentrating ability associated with increased urinary levels of protein, glucose, and electrolytes. Consequently, the kidneys provide a good postmortem diagnostic sample for toxins, and urinalysis can provide objective information about the nature of disease (see Chapter 20). The clinician can assume that a degree of damage is occurring during shock, septicemia, dehydration, or toxemia and should take preemptive steps in preservation and protection of renal function during case management. Changes in blood flow or oxygen delivery to the kidney cause renal insufficiency, potentially leading to acute or chronic renal failure. Dehydration, heat stress, severe rumen bloat, sepsis, and anemia result in physiologic and metabolic changes leading to kidney dysfunction due to decreased cardiac output and renal vasoconstriction and dilation.
Vasopressors and inotropes can be instituted as adjunct therapy but need to be administered as carefully calibrated constant-rate infusions (CRIs); ranges of therapeutic efficacy are wide as well as widely debated, and scientific data for use in small ruminants are largely extrapolated from other species including humans. Much of the human literature, however, is based on information gained from sheep models of disease. In a regimen developed in clinical practice based in part on information gathered from other sources,2 persistent oliguria or anuria can be treated using intravenous dopamine (2 to 5 μg/kg/minute), dobutamine (5 to 10 μg/kg/minute), and a combination of norepinephrine (0.4 μg/kg/minute) plus dobutamine (5 μg/kg/minute). Of note, the use of dopamine alone in cases of ARF may not be as beneficial as was once thought, and additional potential adverse effects have been discovered.3,4
1. Sankarappa E.V., Rao P.R. Renal lesions in sheep and goats in Andhra Pradesh. Indian Vet J. 1982;59:705-708.
2. Corley K.T.T. Inotropes and vasopressors in adults and foals. Vet Clin North Am Eq Pract. 2004;20:77-106.
3. Trim C.M., Moore J.N., Clark E.S. Renal effects of dopamine infusion in conscious horses. Equine Vet J Suppl. 1989;71:24-28.
4. Kellum J.A., Decker J.M. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526-1531.
Acute Renal Diseases
Infectious Diseases
Clostridium perfringens Type D
Disease syndromes caused by Clostridium perfringens type D are referred to as enterotoxemia, overeating disease, and pulpy kidney disease. The 2001 U.S. Department of Agriculture (USDA)-sponsored National Animal Health Monitoring System (NAHMS) sheep survey revealed that 38.8% of sheep flocks had suspected or confirmed cases of enterotoxemia, with 30.9% confirmed by veterinary or laboratory examination, in the previous three years.1 Enterotoxemia most commonly is seen in young, growing animals consuming diets high in rapidly fermentable carbohydrates. High milk or starch content allows for excess colonization of the jejunum with C. perfringens type D, which produces alpha and epsilon toxins,2,3 of which epsilon is the more significant in disease. Epsilon toxin is activated in the intestine and is systemically absorbed, resulting in increased capillary permeability from a loss of endothelial integrity,2 and an influx of protein and fluid occurs in the organs and body cavities. Sheep more often experience the systemic form of the disease, characterized by edema throughout the body, including the brain, lungs and kidneys, often resulting in acute death, whereas goats more often are affected by hemorrhagic enterocolitis.4,5 The systemic form most often seen in sheep commonly results in acute death, but live animals may exhibit seizures, blindness, recumbency, dyspnea,6 and other signs consistent with fluid accumulation in and around organs.
At necropsy, visceral edema, serosal hemorrhage, and cavitary effusions may be present, but death from C. perfringens type D infection also may result in no gross lesions. The cortices of the kidney may be softened and show subcapsular petechiae.6 Epsilon toxin promotes liver glycolysis, resulting in hyperglycemia and glucosuria, making dipstick evaluation of bladder urine a useful test for investigation of acute death in lambs and kids. After experimental infection in sheep, ileum has been found to be the best sample for isolation of epsilon toxin by enzyme-linked immunosorbent assay (ELISA).7 Histopathologic examination of the brain reveals microangiopathy with protein surrounding the arteries and veins, which is pathognomonic for C. perfringens type D infection,6,8 but no lesions may be seen in the kidneys.8 Negative results on testing for C. perfringens type D by any of these means does not necessarily rule out the pathogen as the cause of disease.6,8
Prevention of C. perfringens type D infection is of utmost importance and should include management using vaccination and gradual dietary adaptation. Bacterin toxoids as well as antitoxins are commercially available. Antitoxins are most useful in outbreak situations, because they provide rapid passive immunity, but preemptive use of bacterin toxoids for prolonged, active immunity is preferred for protection against systemic disease. It is recommended that ewes be vaccinated using a C. perfringens type D toxoid–containing vaccine 3 to 4 weeks ante partum, which provided passive protection in lambs up to 12 weeks of age.9 No benefit has been seen with vaccination of lambs before the age of 6 weeks,9 so a potential recommendation is to vaccinate lambs at 6 to 10 weeks of age, with a booster vaccination given 1 month later. Although the vaccine is readily available, inexpensive, and effective, currently only 48.4% of sheep producers in the United States vaccinate breeding or replacement ewes and 66.9% vaccinate nursing lambs and 44.8% vaccinate feeder lambs after weaning, demonstrating a need for producer education based on available research.9
Leptospirosis
Sheep and goats may become infected by a number of serovars of Leptospira interrogans, resulting in several clinical syndromes, bacterial clearance, or a subclinical carrier state.10 The kidneys may become damaged from leptospirosis through hemolysis and interstitial nephritis.
Infected urine is the primary source of infection, with animals obtaining the bacteria from contaminated water or the urine of herdmates, wildlife, rodents, or other domestic animals.11 In one study of experimental infection of sheep with L. interrogans serovar Pomona, clinical disease occurred 34 days after experimental infection.12 Because the bacteria can penetrate intact mucous membranes and because leptospirosis is considered to be the most widespread zoonosis in the world,13 it should be respected as an occupational hazard for veterinarians, staff, and livestock producers.
Animals presented with leptospirosis may show general malaise, fever, icterus, anemia, azotemia, and hemoglobinuria.14 Hemolytic changes in blood analysis are seen 4 to 8 days after infection.14 Total white blood cell counts are often elevated with a neutrophilia.15 A positive result for hemoglobin may occur 3 to 8 days after infection.14 Urine sediment exam may show cellular or proteinaceous tubular casts. The herd or flock history may reveal reproductive manifestations as well, including infertility, abortions, and stillbirths. On necropsy, the carcass is icteric,14 and the kidneys appear dark red and swollen, with pale foci in the cortices, and the liver often is yellow or copper-colored.16 Histopathologic examination reveals a diffuse acute or chronic interstitial nephritis,15,17 and organisms may be observed. Loss of the brush border is a common feature, and necrotic epithelial cells may be seen within the tubules.12
Diagnosis is based on increasing serologic titers in the acute and convalescent periods, using the microscopic agglutination test (MAT),18 complement fixation (CF) test, or ELISA. CF antibodies are short-lived (13 to 18 weeks), whereas MAT antibodies can be detected for longer periods after infection.14 On urine, polymerase chain reaction (PCR) assay, darkfield microscopy, or culture may be used. PCR assay is preferred, because darkfield microscopy has yielded false-negative results in infected animals,14 and culture of urine generally is unrewarding owing to difficulties in growth in artificial media and intermittent shedding.19 Histopathologic analysis and immunofluorescent antibody (IFA) testing20 may identify organisms in renal tissue. One study has determined that, in herd or flock situations, using MAT for herd-level screening, followed by urine PCR assay is suitable for identification of carrier animals.19
Several serovars are reported in small ruminants including L. interrogans serovars Pomona, Hardjo, Grippotyphosa, Icterohemorhagiae, Canicola, and Bratislava.18-,23 L. interrogans serovar Pomona appears to be the most commonly associated with interstitial nephritis and hepatic centrilobular necrosis.12,17 It also has been shown to cause severe hemolytic anemia in lambs.15,17 In one case in lambs, the kidneys were negative for leptospiral organisms, but a rising titer to the Pomona serovar was observed.15 Ewes administered the hemolysin of L. interrogans serovar Pomona experienced a reduction in hemoglobin levels to 57% of the normal range within 48 hours and had lesions similar to animals infected with the whole organism.16 Necropsy findings included placental separation and autolysis of caruncles and cotyledons in some pregnant ewes16 (see Chapter 20). Pigs are the natural reservoir host of L. interrogans serovar Pomona.
L. interrogans serovar Hardjo is host-adapted to cattle17 and sheep.22,24 It has also been reported in a sheep found acutely dead23 with organisms found in the renal tubular epithelium and tubular lumen. Flockmates of this animal were seropositive against serovar Hardjo, with small numbers seropositive against other serovars.23 One study comparing the hemolytic properties of three serovars of leptospires found serovar Hardjo to be more hemolytic than serovar Pomona.21 Sheep with renal infections from serovar Hardjo may not harbor the bacterium in their reproductive tracts,24,25 whereas sheep experimentally infected with serovar Hardjo-bovis show renal localization and harbor the bacteria up to 242 days after infection.25 Treatment of leptospirosis consists of intravenous crystalloid fluid therapy combined with blood transfusion in clinically anemic animals. Because of the difficulty in culturing the organism, antimicrobial therapy is based upon anticipated spectrum of coverage. In cattle shedding leptospires in the urine, the following antibiotic regimens were shown to clear urinary shedding of organisms: oxytetracycline (20 mg/kg IM as a one-time dose), tilmicosin (10 mg/kg SC as a one-time dose), dihydrostreptomycin-penicillin G (25 mg/kg IM once), or ceftiofur sodium (1-2.2 mg/kg IM once daily for 5 days, or 20 mg/kg IM once daily for 3 days). It should be noted that tilmicosin is toxic to goats and should only be used in sheep.26,27 Vaccination for control of leptospirosis may be useful in reducing urinary shedding, but should not be relied upon for protection from disease. Bovine-labeled vaccines are commonly used and suffer from questionable efficacy and duration of immunity even in label species.28–30 In designing management plans for leptospirosis, consideration should be given to biosecurity for new additions, control of access to wild and domestic animals, and the accessibility of potentially contaminated water sources.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree