Chapter 7 Diseases of the Respiratory System
Anatomy
Clinically significant upper airway structures in the small ruminant include the frontal and maxillary sinuses, pharynx, larynx, and trachea. The nasopharynx is the primary path for respiration, but oral respirations are anatomically possible, and “panting” occurs under some fairly normal conditions such as high ambient temperature. Laryngeal structure is similar to that in other species, with small V-shaped vocal folds just caudal and ventral to the arytenoid cartilages.1,2 The retropharyngeal lymph node is located dorsocaudal to the pharynx and can compress the larynx or trachea when enlarged or abscessed. The trachea runs down the ventromedial aspect of the neck from the larynx to the bronchial bifurcation in the thorax. It is composed of incomplete tracheal rings connected by a membranous wall. The tracheal diameter in small ruminants generally is smaller than might be expected and changes at the thoracic inlet: In goats the trachea narrows, whereas in sheep it enlarges.2
In the thorax, the trachea bifurcates into two main bronchi. Just cranial to this bifurcation a separate bronchus branches out to the right cranial lung lobe. The major lung divisions include left and right cranial lobes, each with a cranial and caudal part; the right middle (cardiac) lobe; the right accessory lobe; and the left and right caudal (diaphragmatic) lobes. When enlarged, the mediastinal lymph nodes and thymus may compress or shift the thoracic trachea or lung. The caudal lung border is demarcated by the sixth rib ventrally, by the seventh rib at the lateral midthorax, and by the eleventh rib dorsocaudally. The intercostal vessels and nerves run caudally along each rib, and care should be taken to avoid these structures during thoracocentesis or biopsy procedures.2
Physiology
The respiratory system permits reoxygenation of pulmonary venous blood and release of carbon dioxide formed by cellular respiration. Effective respiration requires both alveolar ventilation and gas diffusion across the respiratory membrane; together, these two processes can be quantified by the ventilation-perfusion ratio, which may be altered during disease. Alveolar ventilation occurs through movement of gas from the terminal bronchioles and depends on inspiratory tidal volume and expiratory functional reserve in addition to respiratory rate. Anatomic dead space (e.g., nasal passages, pharynx, trachea, bronchi) does not contribute to alveolar ventilation. Once in the alveolus, respiratory gases must diffuse between the lung and capillaries. Gas movement across membranes is affected by the diffusion coefficient of the gas, the thickness of the septum, and the surface area available for diffusion. Because carbon dioxide diffuses much more readily than oxygen and is the direct stimulus for respiration, hypoxia may occur without significant increases in respiratory rate. Alveolar septum thickness can be increased by edema and fibrosis. Surface area can be physically decreased by consolidation and emphysema or physiologically reduced by alteration in the ventilation-perfusion ratio stemming from increased physiologic dead space or shunting of blood away from ventilated alveoli.3
Significant innate immune defenses are present in the lung. The sneeze and cough reflexes forcibly expel large particles and irritants from the upper airway. Nasal hairs and air turbulence over the nasal concha will filter out airborne particles as small as 6 μm. Gravitational precipitation will filter smaller particles (1 to 5 μm) in the small bronchioles. Mucociliary clearance efficiently moves trapped particles to the pharynx, where they are either swallowed or coughed out. This system is formed by mucus-producing goblet cells and ciliated epithelial cells that line the respiratory tract from the nasal passages to the terminal bronchioles. Once in the alveoli, particles larger than 0.5 μm will come to lie against the alveolar wall and be cleared by alveolar macrophages or the lung lymphatic system. Particles less than 0.5 μm in size remain suspended and will be exhaled without consequence.4
Diagnostic Approaches
Physical Examination and Auscultation
A thorough and unbiased physical examination is the most important component of the diagnostic evaluation of small ruminants presented for abnormalities of the respiratory tract. Without a complete physical exam, important primary or secondary physiologic problems may be missed, and the diagnostic plan may be incomplete or result in failure to obtain a definitive diagnosis.
The physical exam should be conducted in a systematic manner and must include all aspects of the respiratory system. Before restraining the animal, the clinician should spend a few minutes observing its attitude, stance, respiratory rate, and respiratory pattern from a distance, because significant elevations in respiratory rate and pattern can occur after capture and restraint, particularly in animals that are less socialized. In consequence of the flocking instincts of sheep and goats, animals observed to be standing apart from the rest of the flock or herd are likely to be significantly ill. Once the animal is caught and restrained, the practitioner should begin by evaluating the respiratory system starting at the head (see Chapter 1). The nares should be examined for evidence of serous, mucopurulent, or hemorrhagic discharge from one or both nostrils (Figure 7-1). Unilateral nasal discharge may provide significant localization of a lesion and should be noted on the examination form. Both nares should be accessed for patency by placing either a small cotton ball or a mirror in front of the nose and observing for movement or fogging, respectively. The remainder of the head should be evaluated for evidence of facial deformity or soft tissue swelling indicative of a localized lesion. The pharyngeal area should be palpated, with particular attention paid to the local lymph nodes. When possible, the palpation should include an attempt to feel the area lateral and dorsal to the pharynx by placing a hand alongside the trachea and palpating with gentle dorsal pressure. This area is a common site for retropharyngeal abscesses (often caused by Corynebacterium pseudotuberculosis), which may result in considerable respiratory stridor and effort. The extrathoracic trachea should be palpated from the pharynx down to the mediastinal entrance for any evidence of stricture, dilatation, or external compression. During this portion of the evaluation, occasional gentle squeezing pressure should be applied to the trachea, to determine how easily coughing can be induced. The mediastinal opening is another area that warrants palpation for evidence of space-occupying lesions or tracheal deviation associated with such findings.
After completion of the auscultation exam, several additional pieces of information should be collected. The rectal temperature will reveal whether the animal is febrile, normothermic, or hypothermic. The presence of fever may provide additional evidence of an inflammatory process that may warrant additional diagnostic effort. Additionally, the nutritional status of the animal should be evaluated, because immune dysfunction is more common in young animals with less than adequate reserves of body fat. This assessment is perhaps best performed by body condition scoring of multiple animals in the same management group. Finally, the practitioner should spend some time evaluating the environment in which the diseased animal is housed. Environments with poor ventilation, drafts, dust, or high stocking densities may predispose resident animals to the development of respiratory disease; in such instances, appropriate treatment may require addressing the environmental conditions.
Diagnostic Procedures
Radiography
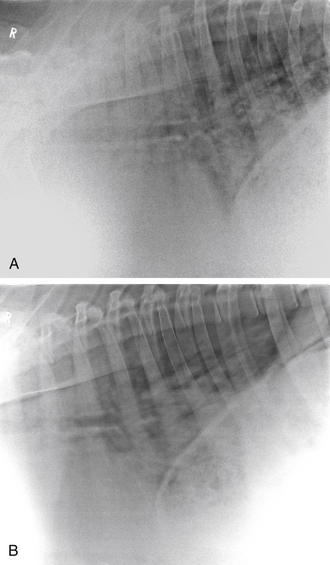
Radiographs of the thorax, neck, or head often are required and can be of significant diagnostic benefit. Radiographs can easily be obtained using portable radiographic equipment commonly available to veterinary practitioners. When unilateral nasal discharge or facial deformities are observed during the physical exam, radiographic evaluation with both lateral and dorsoventral views of the head may elucidate the etiology. In many instances, nasal foreign bodies or sinusitis can be confirmed on the basis of the radiographic interpretation of the head views. Similarly, radiographs of the neck may provide additional evidence of tracheal compression or retropharyngeal masses that may be associated with coughing in affected animals. Thoracic radiographs can be obtained with the animal either standing or in lateral recumbency, depending on the facilities available to the practitioner (Figure 7-2, A and B).
Lung field consolidation can be readily identified by observing radiographic opacities in the cranial ventral lung fields, and mediastinal masses, often associated with caseous lymphadenitis abscesses, generally are revealed as a line of masses of increased density coursing through the thorax at the level of the trachea. In rare instances, a thymoma may result in the appearance of a mass cranial to the heart that gives the appearance of the animal’s having two hearts.
Ultrasound Imaging
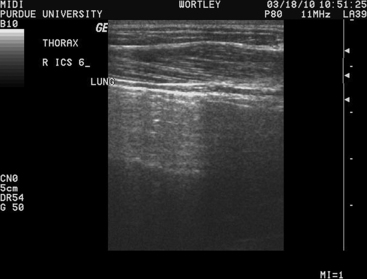
Ultrasound imaging also can provide useful information in evaluation of the thorax. The clinician should become familiar with the appearance of normal aerated lung, allowing rapid identification of areas that lack the normal appearance. Normal lung is recognizable by the bright hyperechoic line of the visceral pleura above a classic reverberation artifact induced by the aerated lung. The reverberation artifact is typical of ultrasound waves hitting a gas interface and consists of sequential hyperechoic lines spaced at regular intervals. It is important to realize that any images appear on the screen deep to the start of the reverberation artifact are indeed artifacts and not images of the lung parenchyma (Figure 7-3). Once an appreciation for the normal appearance of lung has been achieved, the thoracic exam can be systematically performed. With use of a linear or a curvilinear probe, the probe should be oriented parallel to the ribs in the intercostal space. We prefer to start at the most dorsal aspect of each intercostal space and slowly move downwards to the ventral thorax observing the lung surface along the path. This is repeated in each intercostal space moving caudally. The image quality is maximized by following the natural “lay” of the wool or hair (in a dorsal-ventral direction). As the exam progresses caudally, the diaphragm will come into the image while moving ventrally, often with the adjacent liver filling the space below. With use of this method, the extent of the thoracic lung field can be determined. The three primary lesions that will be observed are parenchymal masses in the lung that are adjacent to the visceral pleura, lung consolidation, and the characteristic “comet tail” lesions associated with pleural thickening and inflammation. The first of these lesions is readily identified by the observation of echodense masses interrupting the normal reverberation artifact of the lung. Such masses can be measured to allow for sequential ultrasonographic examination as a means of assessing treatment success or resolution of the lesion. In our experience, these lesions most commonly are associated with parenchymal abscesses. Consolidated lung is recognized on deeper imaging, beyond the normal lung reverberation. In many instances, the consolidated lung may have an appearance similar to that of liver (“hepatized lung”) or may be seen to contain scattered gas shadows associated with presence of gas in the larger airways or in abscesses. “Comet tails” are recognizable as small, hyperechoic spots with a comet tail–shaped artifact located deep to the spot. These lesions are nonspecific but often are associated with thickening or inflammation of the pleura.
Sinus-Centesis
The technique of sinus-centesis provides the practitioner with an option for collecting representative culture material from a nasal sinus. Owing to the comparatively smaller nasal sinuses of sheep and goats, proper selection of a site for sampling is critical to ensure entry into the sinus cavity. Radiographic assistance in localizing the involved sinus cavity is recommended. If necessary, radiopaque markers can be placed on the skin before exposure to ensure appropriate site selection. Once the site is verified, the area should be clipped and surgically prepared. Raising a small bleb under the skin with lidocaine will provide adequate anesthesia to the external surfaces but will not achieve anesthesia to the periosteum. Thus the animal should be sedated or anesthetized (see Chapter 18). If the goal is to collect a small sample of material for culture, a small-diameter bone pin or heavy-gauge circlage wire can be guided through a stab incision in the skin and used to drill a small hole through the bone. A hypodermic needle can then be introduced into the sinus and a sample aspirated. Samples should be submitted for aerobic bacterial and fungal culture. In cases in which drainage and lavage will be needed, a small sinus trephine can be used to create a large-bore opening into the sinus.
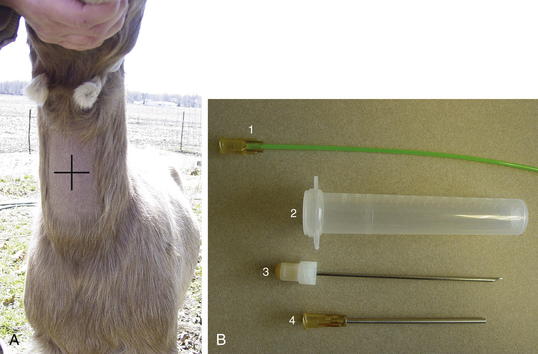
Tracheal Wash
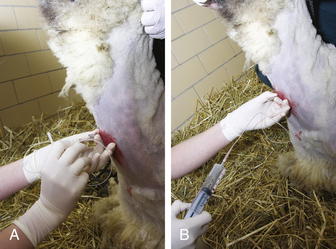
The animal should be standing and adequately restrained for this procedure. Sedation may be warranted. If a fiberoptic endoscope is available (8 to 9 mm diameter), it may be inserted through the nasal passage in some adult sheep or goats. If this is not possible, the endoscope can be passed through an oral speculum. An endoscopic examination may allow visualization of the respiratory tract, identify exudates, and enhance sample collection. If an endoscope is unavailable, a percutaneous transtracheal wash (TTW) procedure can be performed. Use of a commercially available presterilized, complete kit designed for foals, when available, will enhance the ease of this procedure (Figure 7-4, A and B). Alternatively, a hypodermic needle of appropriate size to allow a sterile tube catheter (220 polyethylene) to pass through the bore may be used. On the ventral aspect of the neck, the hair or fiber should be removed over at least a 6-inch square of skin centered on the midline and located roughly one third of the way down the neck from the throatlatch. The trachea should be identified and easily palpated at this level. The site should be disinfected using a standard surgical preparation, and a small bleb can be raised with lidocaine placed under the skin directly over the midline of the trachea in the center of the site. A stab incision should be made through the skin using a scalpel blade. The procedure should be performed using sterile technique. If a commercial kit is to be used, the blunt-tipped needle and associated placement trochar should be identified in the kit, and the clinician should become familiar with their design and use before performing the procedure. The unit should be placed through the skin incision and the tip of the trocar used to palpate the tracheal rings while the operator’s opposite hand is used to stabilize the trachea. The tip of the trocar should be positioned on midline between the tracheal rings and firm pressure applied to facilitate passage of the trochar into the trachea. A slight “pop” may be felt as the tip of the stylet enters the cavity. The trocar should be advanced until it can be felt to fully penetrate the trachea and can be slightly advanced in a ventral direction (Figure 7-5, A). The stylet should be removed and the aspiration catheter passed (using sterile gloves) through the trocar to roughly the level of the tracheal bifurcation. Twelve to 15 mL of sterile saline should be infused into the trachea and a sterile syringe used to apply gentle suction as the catheter is moved back and forth in the trachea (see Figure 7-5, B). The goal is to move the catheter so that its tip is in the pool of fluid created just cranial to the tracheal carina. Although this cannot be visualized, it can be located with practice in a majority of cases. If needed, additional normal saline (another 5 to 10 mL as indicated) can be given to increase the recovery volume. The catheter should be removed, followed by the trocar. In cases in which a needle and polypropylene catheter are used, the needle should be removed from the trachea before the catheter, to minimize the risk of cutting off the distal tip of the catheter during its withdrawal.
Thoracocentesis
Thoracocentesis provides a reliable means of collecting a sample of pleural fluid for diagnostic submission. This is best performed in the cranial-ventral portion of the chest, where pleural fluid pools in the most dependent part of the thorax. While the animal is standing, this area can be evaluated by ultrasound imaging and an appropriate site selected as indicated by presence of fluid and absence of other viscera. The body wall thickness can be measured to assist in determining how deep to advance the needle to acquire the sample. Once collected, the sample should be evaluated for appearance, odor, and turbidity, in addition to being submitted for bacterial culture and cytologic study. The presence of a pungent foul odor often is associated with anaerobic infection, and treatment decisions should consider this possibility.
Upper Airway Disease
Oestrus ovis Infestation
Pathogenesis
Nasal bot infestation is more common in sheep than in goats, and infected goats have a lower larval burden than that typical for sheep.5 Clinical signs during the first spring infestation generally are mild, but disease severity markedly increases during subsequent infestations, probably owing to hypersensitization; goats may acquire immunity after repeated infections.5 The adult Oestrus ovis fly deposits larvae at the animal’s nostrils. The first instar larvae migrate up the nasal passages into the dorsal turbinates and sinuses. There they develop over a 2- to 10-month period to the third instar stage,1,6 return to the nostril, and are sneezed out to pupate in the soil.6 Both first instar larvae and pupae may overwinter.6
Clinical Signs
Irritation from the adult flies will induce avoidance activities such as head shaking, head rubbing, and feet stomping; if the animal’s distress level is severe, grazing activity will decrease.6 Larval passage and development can cause inflammatory rhinitis characterized by sneezing, mucopurulent discharge, and decreased airflow through the nares. Sequelae can include bacterial rhinitis or sinusitis and, infrequently, interstitial pneumonia secondary to antigen aspiration.5
Diagnosis
O. ovis infection is associated with a profuse nasal discharge containing numerous eosinophils and mast cells.5,7 Direct visualization of the bots or mineralized remains may be possible with endoscopic or radiographic imaging. In commercial herds, clinical signs, cytologic examination of the discharge, and response to therapy usually are sufficient to make the diagnosis.
Therapy
Treatment usually is administered for heavy late summer infestations or to kill overwintering bots. Ivermectin (0.2 mg/kg SC) is effective in killing the O. ovis larvae1,6,7 but requires an extended milk withdrawal period: 40 days if administered subcutaneously and 6 days if administered orally (if administered at a higher oral dose of 0.4 mg/kg, an 11-day milk withdrawal is recommended).8 Pour-on eprinomectin (0.5 mg/kg) may be a better choice for commercial dairies because it has been shown to be effective in sheep against nasal bot infestation and has a zero-day milk withdrawal period.9,10
Once the bots are killed, secondary bacterial infections usually will resolve without further intervention. If indicated, however, treatment is with broad-spectrum antimicrobials.7
Other Parasites
In the Himalayas, a nasal leech from standing water pools can cause similar clinical signs. Systemic ivermectin is ineffective, but direct application of ivermectin solution (0.1 mg/mL) to the leech will kill it within a few hours. Wetting the animal’s muzzle will encourage the leech to migrate down to the nostril opening so that the ivermectin can be applied.1
On the Indian subcontinent, Schistosoma nasale infection (“snoring disease”) has been reported as a cause of nasal obstruction from parasite-associated inflammation and tissue proliferation.1,11
Enzootic Nasal Tumor
Pathogenesis
Enzootic nasal tumors are transmissible, sporadically occurring tumors of the nasal passages of sheep and goats.12,13 This condition has been reported in animals as young as 15 and 7 months, respectively,14,15 and is believed to be caused by type D or B retrovirus infection.12,16,17 These tumors can occur unilaterally or bilaterally and are locally invasive but not usually metastatic.13,15 They originate from the olfactory mucosa and ethmoid or nasal turbinates and usually are classified as adenomas, adenopapillomas, and adenocarcinomas.13–15 Other conditions on a differential diagnosis list for nasal masses include lymphosarcoma and fungal granuloma.1
Clinical Signs
The tumor starts as small nodules that grow to form large nodular cystic masses, causing progressive inspiratory dyspnea and secondary emaciation.13,15 Inflammatory polyps may be present near the tumor.15,17 Primary clinical signs include unilateral or bilateral copious seromucous to mucopurulent nasal discharge with inspiratory stridor. Additional signs may include exercise intolerance, decreased airflow and open-mouth breathing, anorexia, head shaking and sneezing, exophthalmos, and bony facial asymmetry.13–15
Diagnosis
A preliminary diagnosis can be made from the clinical signs and findings on sinus percussion. Radiographic or endoscopic imaging may be indicated. Definitive diagnosis requires surgical excisional biopsy.14
Treatment
If enzootic nasal tumor is untreated, death will occur within 90 days of appearance of clinical signs.13,14 Surgical debulking is a palliative option14 but may not be curative.18 The mass can be accessed for excision by creating an I-shaped incision in the skin and then the nasal bones along the dorsal facial midline axis, reflecting the cutaneous and bony flaps, and removing the nasal septum. Profuse hemorrhage is to be expected; epinephrine (1:100,000)-soaked gauze pads can help with hemostasis, and a blood donor should be readily available.18 A temporary tracheostomy may be needed during the surgical procedure and the postsurgical period.14 Herd or flock control of enzootic tumor is difficult in the absence of a serologic test to identify animals with preclinical disease. Enzootic nasal tumors can be spread by nasal discharge; infected animals should be isolated and culled.17
Other Respiratory Infections
Other respiratory infections involved in small ruminant rhinitis include herpesvirus and Pasteurella multocida infections. Herpesvirus infection causes fibronecrotic ulceration of the nasal septum with a marked catarrhal rhinitis, usually accompanied by additional severe systemic signs.1 P. multocida infection causes nasal turbinate atrophy, which can be identified at necropsy by cross-sectioning the head at the level of the first premolar.1 In tropical and subtropical regions, an important consideration in the differential diagnosis for bacterial rhinitis is nasal melioidosis (caused by Burkholderia pseudomallei).1 Respiratory involvement is particularly common in small ruminant species and may include oculonasal discharge, coughing, lymphadenopathy, and pulmonary disease, all characterized by multiple caseous abscesses. Melioidosis is zoonotic and reportable in many parts of the world.19
Sinusitis
Pathogenesis
Sinusitis is a relatively rare condition in sheep and goats, usually related to dehorning infections (with consequent involvement of the frontal sinus) or dental abnormalities (with maxillary sinus involvement). Signs of frontal sinus infection may appear weeks to months after the dehorning process. Multisinus infection can result from nasal bot infestation, neoplasia, facial fractures, and horn injuries.7
Clinical Signs
Indications of sinusitis include drainage from dehorning sites as well as swelling, softening, or deformities of the overlying facial bones. Malodor, unequal airflow, head shaking and rubbing, extension of the head and neck, or head resting or pressing also may be noted. Systemic signs such as pyrexia, anorexia, and lethargy may develop as well, and chronic sinusitis may lead to neurologic symptoms.7
Diagnosis
A presumptive diagnosis can be made from the clinical presentation and findings on percussion. Radiographic imaging is indicated for investigation of recurring or refractory cases. In one instance of chronic sinusitis in a pet goat, computed tomography was used to accurately characterize the lesion.20 An oral exam with the animal under light sedation should be performed if dental abnormalities are suspected. Culture and sensitivity testing of the sinus exudate can help direct antimicrobial selection.
Treatment
Basic therapy involves daily lavage of the dehorning site and sinus with a dilute antiseptic such as 0.1% chlorhexidine. Lavage solution can be introduced through a teat cannula or 16-18 French catheter. Multiple trephination sites may be needed, especially in the highly compartmentalized ovine frontal sinus.7 Trephine holes need to be large enough to establish drainage; 14-gauge needles commonly are used for diagnostic sampling but are too small for lavage. Placement and ease of trephination are facilitated by the softer bone and the bone deformity found in typical chronic sinusitis cases.21 The caudal frontal sinus can be accessed 5 mm from the base of the horn while avoiding the frontal vein in the supraorbital groove; the rostral frontal sinus lies medial to the orbit. Trephination borders for the maxillary sinus are cranial to the orbit, caudal and dorsal to the facial tuberosity, and ventral to the infraorbital foramen.22
Complete resolution may require a couple weeks of daily treatment, because the sinus structure is complex and biofilm development is common. Sheep have been used in experimental models for antibiofilm approaches to sinusitis; early results are promising.23 Animals showing systemic signs should be treated with antibiotics (penicillin, 22,000 IU/kg twice daily) and nonsteroidal antiinflammatories (e.g., flunixin meglumine, 1.1 mg/kg IV twice daily, or ketoprofen, 3.0 mg/kg IV or IM once a day).
Sinusitis may be prevented by bandaging open dehorning sites for 5 to 7 days after the procedure and by gauze-packing extracted tooth sockets.7
Pharyngitis
Pathogenesis
In sheep and goats, pharyngitis typically develops secondary to traumatic injury, with subsequent bacterial colonization. Inciting trauma usually is caused by dosing equipment, rough feeds, or foreign objects. Plastic animal health devices (e.g., dosing and balling guns, stomach tubes, speculum), especially older devices that have been roughened from chewing, are notorious for traumatizing the pharynx. Commonly involved pathogens include Arcanobacterium pyogene, Fusobacterium necrophorum, and C. pseudotuberculosis.7
Treatment
Pharyngitis should be treated with parenteral broad-spectrum antibiotics and NSAIDs. Oral medications and forced tube feeding are contraindicated; a temporary rumen fistula can be placed if nutritional support is needed. Abscesses can be drained into the pharyngeal cavity and flushed with a dilute antiseptic, such as 0.1% or 0.2% povidone-iodine (Betadine).7
Retropharyngeal Abscesses
Although retropharyngeal abscesses can develop in association with pharyngitis, they more commonly are due to C. pseudotuberculosis infection. Clinical signs result from pressure on the pharynx and trachea and include stridor, cough, and difficulty swallowing. Diagnosis is based on clinical signs, palpation, and possibly radiographic imaging. To avoid contamination of the environment, C. pseudotuberculosis abscesses should not be lanced. Surgical removal of the retropharyngeal lymph node is technically possible but difficult owing to the presence of vital anatomic structures in the region. Closed-system lavage along with either intralesional or subcutaneous tulathromycin (2.5 mg/kg) is as effective as traditional methods of lancing, draining, and flushing subcutaneous caseous lymphadenitis abscesses24; this approach may be an option if the retropharyngeal abscess can be accessed percutaneously.
Laryngitis and Tracheitis
Necrotic laryngitis (necrobacillosis, “calf diphtheria”) is caused by invasion by the opportunistic anaerobe F. necrophorum through breaks or ulcers in the laryngeal mucosa. This condition is rare in sheep and goats but is seen more commonly with indoor housing systems and in feedlot environments. Clinical signs include a moist-sounding painful cough, inspiratory dyspnea, difficulty swallowing, and salivation. A presumptive diagnosis usually can be made on the basis of clinical signs; laryngoscopic and endoscopic examinations are warranted with recurring or refractory cases. In cattle, most early cases respond well to broad-spectrum antimicrobial therapy25 and NSAIDs. A temporary tracheostomy may be needed until medical therapy takes effect.
Laryngeal chondritis is characterized by edema, suppuration, necrosis, and abscessation of the arytenoid cartilages. This disease has been described in Texel sheep as well as in cattle and horses.25–27 Breed predilections have been documented, but mode of inheritance is unknown.27 Clinical signs may resemble those of necrotic laryngitis and include increased upper airway noise, dyspnea, cyanosis, and possibly halitosis; if the condition goes untreated, clinical progression and death are expected.26,27 Diagnosis in the live animal requires endoscopic evaluation of the arytenoids. Partial arytenoidectomy has been suggested as a treatment,7,26 but subsequent aspiration pneumonia has been observed in cattle.27 Goulding and associates reported a successful standing permanent tracheostomy in a heifer; the surgery was intended as a salvage procedure, but the heifer was retained and bred successfully.25 If laryngeal chondritis is caught before cartilage necrosis, abscess formation, or granulation, early ovine and bovine cases have been successfully treated with broad-spectrum antibiotics (lincomycin) and dexamethasone.26,27
Laryngeal hemiplegia has been reported in an Alpine goat. No cause was identified on necropsy.1
Tracheitis most commonly is caused by pressure from collars and tethers or may result from airborne irritants such as dust and ammonia.1
Tracheal collapse is a rarely reported congenital condition in the goat. In view of the surprisingly small diameter of goat tracheas, animals in which the condition is suspected should be evaluated by comparison with healthy peers. Clinical signs include stridor, exercise intolerance, and coughing. Affected animals may lag behind their peers in growth and performance.28 One case has been reported in a previously asymptomatic adult goat; clinical onset presumably was triggered by increased respiratory effort secondary to pneumonia.29 Diagnosis is based on recognition of clinical signs and tracheal palpation aided by radiologic or endoscopic examination. Successful treatment in cattle and one kid using surgically implanted prosthetic rings has been described.28
Cilia-associated respiratory bacillus (CAR) is a bacterium that causes tracheitis in laboratory rats and cattle. This bacillus also has been identified in tracheas from goats with chronic caprine tracheitis and in lungs from kids and adult animals with enzootic pneumonia.30,31 The significance of CAR in small ruminant respiratory disease is not yet known.
The viral agent of infectious bovine rhinotracheitis (IBR), although rarely isolated from field cases, is capable of causing tracheitis, cough, and nasal discharge in experimentally infected goats. Goat isolates are indistinguishable from those in bovine cases, and some researchers theorize that goats may be latent carriers. IBR vaccination in goats is not recommended, because it is not clear that the causative organism has an actual role in caprine respiratory disease.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree