Chapter 11 Diseases of the Musculoskeletal System
Examination of the Musculoskeletal System
Sheep and goats are herd animals, which by their nature prefer living and staying in a group. Therefore any examination of these animals on the farm should include initial observation of the entire group if possible. Flock observation probably is less important in the evaluation of traumatic musculoskeletal conditions than when several animals are affected by infectious diseases, parasitism, nutritional disorders, or improper management. The practitioner should look for potential hazards around feeders and other areas of the environment when the herd has a higher-than-expected incidence of fractures or injury. The flock or herd should be observed closely to identify animals that lie down or walk on their knees when their herdmates are moving around. Other clinical problems to look for include difficulty in rising, swollen or enlarged joints, lameness, and abnormal stance.
Related Anatomy
Sheep and goats, like cattle, are members of the Bovidae family. They join several other even-toed species in the order Artiodactyla. Animals in this order share three skeletal characteristics: the talus has distal and proximal trochleae; the calcaneus and the fibula articulate with each other; and the limb axis divides the fused third and fourth metacarpal-metatarsal bones and the associated digits.1 Sheep have short, blunt spinous processes of the cervical vertebrae, whereas those of goats are longer and pointed, with sharp edges. Small ruminants have 7 cervical vertebrae, 13 thoracic vertebrae, 6 or 7 lumbar vertebrae, 4 sacral vertebrae, and 16 to 18 caudal vertebrae. The presence of 7 cervical vertebrae is a reliable trait in identification. However, variations are not unusual, such as 12 or 14 thoracic vertebrae or 5 lumbar vertebrae. Occasionally, an unusual transitional vertebra that is difficult to classify is found between the thoracic and lumbar vertebrae.1
Congenital Conditions
Myotonia Congenita
Myotonia congenita is a heritable disorder of goats in which the affected animal experiences tetanic muscle contraction when startled. Occasionally the contraction is severe enough that the goat collapses to the ground. Animals in which this phenomenon is observed have been referred to as “fainting goats.” The disorder is inherited as an autosomal dominant trait.1 Some investigators speculate that the variability in clinical signs and intensity of muscle contractions may be related to homozygous versus heterozygous genotype, with homozygosity more likely than heterozygosity to be associated with clinical manifestations.1 The condition closely resembles a form of myotonia congenita in humans, and the animal disease has therefore been used as a research model for the human disease.
The condition is caused by a mutation in the voltage-dependent chloride channel in skeletal muscle that leads to hyperexcitability of the sarcolemma and delayed relaxation of contracted muscle.3 Histochemical and ultrastructural abnormalities have been documented in goats with myotonia congenita.1,2
Hereditary Chondrodysplasia (Spider Lamb Syndrome)
Hereditary chondrodysplasia, or spider lamb syndrome, is an inherited musculoskeletal condition that is seen primarily in the Suffolk and Hampshire breeds.4 Clinical signs may be present at birth, or affected lambs initially may appear normal, only to have the severe skeletal abnormalities develop by the age of 6 weeks.5 This later presentation may be associated with longer legs with angular deviations, shallower bodies, and narrower chests than normal lambs,5 and these animals display the expected radiographic abnormalities associated with this condition at birth. Skeletal abnormalities exhibited by affected lambs vary in severity and type. Chondrodysplasia is evident in the skull, sternum, appendicular skeleton, and vertebrae.
On radiographic evaluation, the dorsal silhouette of the skull may be rounded, the occipital condyles may be elongated (occasionally with cartilage erosion), and thickening of the occipital bone between the condyles and the poll may be evident. The sternebrae may be of abnormal size and shape. The sternum often is misaligned, dorsally deviated, and not fused across the midline. The scapula and olecranon usually have more cartilage and less bone distally than normal. Several islands of ossification near the anconeal process typically can be seen on flexed lateral radiographs of the elbow in animals with this syndrome. The distal physis of the radius is flared, and angular limb deformities are common. The forelimbs generally are more severely affected than the hindlimbs. Erosion of articular cartilage is common if the lamb survives for a few months. The vertebrae commonly have abnormal and excessive cartilage. Vertebral body abnormalities may contribute to scoliosis or, less commonly, kyphosis.5 On histopathologic examination, the typical osseous lesion is manifested as uneven growth cartilage. The pathologic changes are found by the end of the second trimester of gestation.5
Spider lamb syndrome is caused by a mutation in fibroblast growth factor receptor 3 (FGFR3) that leads to excessive skeletal growth.6 Although inheritance initially was considered to follow an autosomal recessive pattern with complete penetrance but variable expression, genetic testing has led to a suggestion of a codominant pattern. Heterozygotes occasionally are affected with spider lamb syndrome but more typically have a phenotype close to normal but with longer bones than in animals without the mutation.6 Carriers were difficult to identify until a DNA test became commercially available. The incidence of spider lamb syndrome has greatly decreased since the test became available.7
Arthrogryposis
Inherited arthrogryposis has been reported in Suffolk8 and Corriedale9 sheep. It appears to follow an autosomal recessive pattern, and a site on chromosome 5 has been identified as the likely genetic locus.10
Polydactyly
By definition, polydactyly is a congenital anomaly in which extra digits are present. It is seldom seen in sheep and goats. The condition is certainly heritable in cattle and probably heritable in pigs, where cleft palate may concurrently be seen. Polydactyly is suggested to be heritable in horses. One report of polydactyly in goats describes an affected female that was sired by a male with polydactyly.11 Polydactyly usually has only cosmetic consequences for affected animals but may be associated with serious gait abnormalities in some instances. The practitioner must thoroughly examine animals with gait abnormalities to determine whether the lameness is because of some other anomaly or clinically significant lesion. Radiographs are necessary to assess the anomaly fully and to determine any treatment to be rendered.
Patella Luxation
Surgery usually is indicated for young animals with congenital patella luxation. Most young animals respond well to imbrication of the fibrous joint capsule and overlying fascia on the side opposite the direction of patella luxation. However, the veterinarian must fully evaluate the limb preoperatively and assess the joint at surgery. Some severe cases may require trochleoplasty or tibial crest osteotomy and relocation. Detailed descriptions of the more complex stifle surgeries are available in small animal surgery texts.12
Affected animals should be thoroughly examined for other congenital abnormalities. Specifically, severely affected newborns may not be able to stand and suckle. Therefore failure of passive transfer and associated illness may become more significant to the health of these animals than even the primary patella luxation. In mild cases of luxation, especially if the condition is unilateral, small ruminants may compensate well enough biomechanically that the condition goes undiagnosed until they present in adulthood with lameness caused by luxation or degenerative joint disease caused by intermittent luxation. One report of development of patellar luxation as late as the age of 2 years in sheep with common bloodlines suggests some genetic predisposition to the condition.13 Adult animals also may exhibit acute lameness as a result of traumatic patella luxation. Surgical treatment of adults tends to be more involved in that orthopedic implants such as screws and wires may be required to secure the patella in the normal position. Older animals also may require wedge trochleoplasty or tibial tuberosity transposition in addition to imbrications and fascial release.13 The prognosis for a return to soundness is not good compared with that in neonates treated for congenital luxation.14,15
Spastic Paresis
Spastic paresis has been described in pygmy goats.16 Affected goats suffer constant contraction of the gastrocnemius muscles in the hind legs. The contraction produces extension of the tibiotarsal joint and arching of the back. Clinical signs are not significantly different from those described in several breeds of cattle.17–19 This condition is suspected to be inherited, but the exact mode of transmission is unknown. No lesions have been noted in the spinal cord, tibial or peroneal nerve, or gastrocnemius muscle. The clinical signs appear to be caused by a defect in the myotactic reflex that results in an overstimulation or relative lack of inhibition of the efferent motor neurons.16
Carpal Contracture
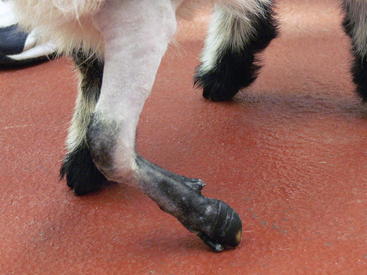
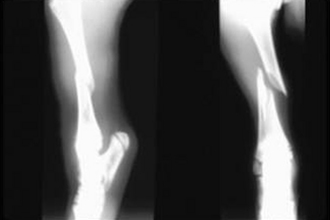
Carpal contracture occasionally can manifest as a congenital condition in kids and lambs. Many will respond very quickly to treatment with splints and bandages. Radiographic examination of the limbs is recommended to identify any osseous lesion that may contribute to the flexural deformity. Careful palpation of the limb while attempting to straighten it will frequently identify the structures under tension. Tenotomy of the restrictive structure may relieve the flexural deformity (Figures 11-1 and 11-2). Flexural deformities may develop in older animals after an injury that leads to abnormal weight bearing. This secondary flexural deformity often will involve fibrosis of the joint capsule and seldom responds to tenotomy. Some cases will not resolve despite a release incision of the joint capsule and all flexural structures between the skin and joint capsule.
1. Bryant S.H., Lipicky R.J., Herzog W.H. Variability of myotonia signs in myotonic goats. Am J Vet Res. 1968;29:2371.
2. McKerrell R.E. Myotonia in man and animals: confusing comparisons. Equine Vet J. 1987;19:266.
3. Beck C.L., Fahlke C., George A.L. Molecular basis for decreased muscle chloride conductance in the myotonic goat. Proc Natl Acad Sci U S A. 1996;93:11248-11252.
4. Rook J.S., et al. Diagnosis of hereditary chondrodysplasia (spider lamb syndrome) in sheep. J Am Vet Med Assoc. 1988;193:713.
5. Oberbauer A.M., et al. Developmental progression of the spider lamb syndrome. Small Rumin Res. 1995;18:179.
6. Beever J.E., et al. A single-base change in the tyrosine kinase II domain of ovine FGFR3 causes hereditary chondrodysplasia in sheep. Anim Genet. 2006;37:66-71.
7. Jolly R.D., Blair H.T., Johnstone A.C. Genetic disorders of sheep in New Zealand: a review and perspective. N Z Vet J. 2004;52:52-64.
8. Doherty M.L., Kelly El.P., Healy A.M. Congenital arthrogryposis: an inherited limb deformity in pedigree Suffolk lambs. Vet Rec. 2000;146:748-753.
9. Whittington R.J., et al. Congenital hydranencephaly and arthrogryposis of Corriedale sheep. Aust Vet J. 1988;65:124-127.
10. Murphy A.M., et al. Linkage mapping of the locus for inherited ovine arthrogryposis (IOA) to sheep chromosome 5. Mammal Genome. 2007;18:43-52.
11. Al-Ani F.K., Hailat N.Q., Fathalla M.A. Polydactyly in Shami breed goats in Jordan. Small Rumin Res. 1997;26:177.
12. Hulse D.A., Shires P.K. Textbook of small animal surgery. Philadelphia: WB Saunders; 1985.
13. Shettko D.L., Trostle S.S. Diagnosis and surgical repair of patellar luxations in a flock of sheep. J Am Vet Med Assoc. 2000;216:564.
14. Baron R.J. Laterally luxating patella in a goat. J Am Vet Med Assoc. 1987;191:1471.
15. Gahlot T.K., et al. Correction of patella luxation in goats. Mod Vet Pract (May):418. 1983.
16. Baker J., et al. Spastic paresis in pygmy goats. J Vet Intern Med. 1989;3:113.
17. Leipold H.W., et al. Spastic paresis in beef shorthorn cattle. J Am Vet Med Assoc. 1967;151:598.
18. Thomason K.J., Beeman K.B. Spastic paresis in Gelbvieh calves: an examination of two cases. Vet Med. 1987;82:548.
19. Harper P.A.W. Spastic paresis in Brahman crossbred cattle. Aust Vet J. 1993;70:456.
Traumatic Conditions
Predator Attack
Sheep and goats are of the stature and disposition to make them susceptible to predators. In the United States, predation accounts for about 37% of sheep and lamb losses, primarily involving attacks from coyotes and dogs.1 Small ruminants seldom survive attacks by wild carnivores. However, veterinarians are sometimes called to treat survivors of attacks by domestic animals or interrupted attacks by wild animals. These survivors often ultimately die because of either lethal injury to internal organs or physical exhaustion from the chase and the attack. A veterinarian treating animals that survive the initial trauma may face a significant challenge. Although skin wounds are quite obvious after the animal is thoroughly examined and clipped, injuries to deeper structures and serious myopathy are more difficult to assess.
Attacking predators tend to “go for the jugular,” which leads to a concentration of wounds in the head and neck area. The associated injury to the great vessels usually is obvious and often fatal. Tracheal puncture can cause respiratory difficulties leading to subcutaneous emphysema. Subcutaneous emphysema also can result from the undermining skin wounds alone, making diagnosis of tracheal perforation difficult in some cases and adding to the difficulty of detecting a tracheal wound. Perforation of the esophagus is common. Esophageal injury may lead to abscess formation and tissue necrosis as a result of contamination of surrounding tissues by esophageal contents. An abscess may physically impinge on the airway, making swallowing difficult. Neurologic damage from the primary injury or damage caused by abscessation may inhibit normal function of the soft palate.
Fractures
The most commonly treated fractures occur in the metacarpal and metatarsal bones.22 These fractures usually are treated successfully with casting. Fractures of the distal half of the metacarpal and metatarsal bones often respond well to use of lower limb casts that incorporate the foot and extend proximally to a point just distal to the carpus or tarsus respectively. Proximal or comminuted metacarpal and metatarsal fractures may require full-limb casting with or without transfixation pins to stabilize the fracture properly and prevent collapse.
Many fractures of the carpus or tarsus also respond to treatment with a full-limb cast.23 However, these injuries are often associated with contamination of the joint, and the incidence of septic arthritis is high. Septic arthritis requires more intensive antibiotic therapy, as well as local treatment provided through a window in the cast. One frequent complication with using treatment windows in casts is “window edema.” The cast window should be cut out as one piece. Edema can be minimized by securing this piece in the window with tape between treatments. The management of carpal or tarsal fractures with concomitant septic arthritis is difficult. Ankylosis of the joint often results even if successful fracture healing occurs.23
Radius fractures must be evaluated individually to determine the best mode of treatment. Fractures of the distal radius may respond to a full-limb cast. Proximal radius fractures may heal better with the use of an external fixator, a transfixation cast, or possibly a modified Thomas splint. Use of splints may be very applicable for neonates, and the splint need stay in place for only 2 to 4 weeks in most instances.24 Some radius fractures may be best treated with internal fixation using plates and screws. Internal fixation is seldom required, however, and often is not economically feasible in small ruminants. If a splint is used for a radius fracture, it should extend from the ground or fetlock to the elbow and preferably above it.24
Treatment decisions for tibia fractures are very similar to those for radius fractures. Distal fractures heal well with full-limb casting.25 The fractured tibia responds well to an external fixator or in larger goats (over 60 pounds) a transfixation full-limb cast (Figure 11-3). Fractures of the humerus and femur occur less frequently in small ruminants.22 Humerus fractures often heal with stall rest alone. However, the distal limb frequently suffers carpal contracture, rendering the animal unsound regardless of fracture healing. Femoral fractures may heal if the limb is taped to the abdomen in a modified Ehmer sling (made of tape placed in figure-eights around the limb). This method is less costly but is still effective in young or lightweight animals.24 Fractures of the humerus and femur frequently heal better with internal fixation using plates and screws or intramedullary pins. The mode of internal fixation depends on the complexity of the fracture and the experience of the veterinarian. Financial considerations may dictate the use of intramedullary pins rather than plates and screws when possible.
Fractures in other areas such as the scapula and pelvis can be treated much as they are in the dog. Small ruminants usually are good orthopedic patients because of their relatively small size and ability to maneuver well on three limbs. Often pelvic or scapula fractures heal if the animal is confined for 3 to 6 weeks.24 The veterinarian can form a plan for treating unusual orthopedic injuries in small ruminants by applying principles of small animal orthopedics and considering cost-benefit decision-making processes for food animal medicine.
Mandible fractures may occur in small ruminants that have been kicked by a large animal such as a horse or cow and those that have caught the rostral mandible in a fence or some other object. A kick injury may result in any number of fracture configurations; the veterinarian must refer to information on small animal fundamentals to determine whether plates, wires, or pins are the most appropriate surgical stabilizers. Frequently external fixators can be used to treat mandibular fractures. In our own practice, we use cortical bone screws placed in the mandible through stab incisions, leaving the screw to protrude about 2 to 4 cm out of the skin. Then acrylic is made to fit over the screw heads and act as connecting bars of an external fixator. The screws provide better stability in the mandible than that afforded by transcortical pins, and the acrylic allows more liberty in screw placement than that permitted by traditional connecting bars. Rostral fractures may involve mostly teeth and soft tissues but very little bone. They often cause loss of teeth but minimal instability. Therefore the veterinarian may wish to debride the area, institute antibiotic therapy, and recommend appropriate modifications to the animal’s diet. If the mandibular fracture occurs between the incisors and the cheek teeth, it may be stabilized by securing wires from the rostral mandible to the cheek teeth.26,27 Animals with these types of fractures require nutritional support, provided either orally or parenterally (see Chapters 2). Many of these animals can be fed a moistened pelleted diet.
Occasionally digit or leg amputation is required to treat septic conditions, fractures, or luxations. Amputation can be done with the animal under general anesthesia or with use of sedation and local anesthesia (see Chapter 18). For digit amputation, a tourniquet should be applied proximal to the fetlock after the surgical site is prepared in an aseptic manner. A circumferential skin incision is made just proximal to the coronary band. The surgeon may then make two incisions perpendicular to the circumferential incision (one dorsal and another palmar or plantar) to create a skin flap that is elevated to allow amputation with Gigli wire. We prefer to make one incision over the abaxial aspect of the affected digit perpendicular to the coronary band to create an inverted T incision. The two flaps of the inverted T can be undermined to allow the passage and crossing of the Gigli wire. The amputation should be completed on an angle at the distal aspect of the proximal phalanx (Figure 11-4), with removal of all of the articular cartilage and synovial membrane of the proximal interphalangeal joint; the interdigital ligaments are left intact to provide stability to the fetlock. The corners of the flaps of the inverted T can be trimmed to minimize dead space when the surgical site is closed. The site can be closed completely if the amputation is performed as a treatment for fracture or luxation. However, if infection is present in the form of septic arthritis or osteomyelitis, the clinician should consider the advantages of drainage facilitated by partial closure. With either closure, a bandage should be placed on the foot to aid in hemostasis before the tourniquet is removed. The bandage should be changed as needed until the incision site has healed. The use of broad-spectrum antibiotics (oxytetracycline 10 mg/kg given IV or intramuscularly [IM] twice daily, or 20 mg/kg once a day every 48 hours) and antiinflammatory drugs should be considered.
Cast
As discussed earlier in this section, casting is a primary treatment option for fixation of fractures. The clinician should prepare the limb for cast application by removing any organic debris to ensure that the leg is clean. Cotton or gauze sponges should be placed in the interdigital space to prevent pinching of the interdigital skin within the cast by the hooves. Orthopedic felt or gauze sponges should be placed over the dewclaws to provide padding; however, holes should be cut to allow the dewclaws to protrude. Without this precaution, pressure from the cast over the dewclaws can cause skin ulcerations and may even result in dewclaw sloughing. The clinician then applies a double layer of stockinette to the limb and places a strip of orthopedic felt around the limb where the most proximal part of the cast will end. We prefer to put this proximal felt between two layers of stockinette so the felt is encased in the stockinette when it is rolled down over the felt during application of the cast. However, others place the felt beneath the stockinette. Other padding materials may be used according to preference, but the clinician should remember that the relatively small size of many sheep and goats demands that the cast not be overly heavy or bulky. We believe that no padding beyond the previously mentioned interdigital cotton, orthopedic felt, and stockinette is necessary to prevent skin ulceration under a properly applied cast. If the wool of heavily wooled animals is not clipped, it may act as excellent padding.24 An exception in which additional padding is useful is for very young animals, which are likely to experience significant growth while in the cast and tend to be more prone than adults to development of cast sores.
Use of transfixation pins adds stability in cases in which cast immobilization alone is not adequate.22 Transfixation pins help immobilize proximal fractures in ways that casting alone does not. Some comminuted distal fractures will collapse unless transfixation pins transfer the weight away from the distal limb to the pins.28 Application of a transfixation cast often requires general anesthesia, although casting of hind limbs can be done with use of sedation and spinal anesthesia. Pin diameter and placement will depend on animal size, bone diameter, and fracture configuration. The transfixation pins are placed through stab incisions using aseptic technique. Intraoperative radiographs are helpful in the placement of the transfixation pins. However, this technique usually is successful even when pin placement is directed by palpation alone. Antimicrobial ointment, such as “Zipp” ointment or neomycin–polymyxin B–bacitracin, can be applied to the skin at the pin sites, which is then covered with gauze sponges. The limb is then prepared as previously described for cast application. The formula for “Zipp” ointment, which has an antibacterial effect lasting as long as 2 weeks, is given in Box 11-1.
External Fixation
External fixators are preferable to simple casts or transfixation casts for stabilization of some fractures of the radius and tibia. Either traditional fixators or modified fixators using cast material to support the transcortical pins work well in small ruminants. Traditional external fixation techniques described for small animals can be used for sheep and goats.29 Standard smooth intramedullary pins can be used successfully for this purpose, but our own preference is for positive-profile threaded pins to provide additional stability. A modified fixator designed to treat calf tibia fractures is less technically demanding to apply than a traditional external fixator30 and allows more flexibility in pin placement. We have found this technique to be most useful in tibia fractures but also of value for management of other fractures. The procedure is performed on a surgically prepared animal, under general anesthesia (see Chapter 18), according to aseptic technique. At least two pins must be placed proximal and two pins distal to the fracture site. The pins can be placed through stab incisions from lateral to medial (type II pins) through the skin on each side. One major advantage of this technique is that a single type I pin can be placed from the dorsal aspect. The type I pin passes through one skin surface and both cortices of the bone, but not through the caudal soft tissues and skin. A second type I pin is not required because the cast material itself connects and stabilizes the pins. This inherent stabilization is a major advantage in fractures (either proximal or distal) in which the fragment size does not allow placement of two type II pins. The pins should be incorporated into a cast as described previously for the transfixation cast and the limb treated with topical antibiotic ointment. This technique incorporates more padding than that used with a standard cast. Cotton or some other padding should be wrapped around the entire length of the tibia. No stockinette or orthopedic felt is required. Fiberglass cast material should then be placed over the length of the tibia to incorporate the pins, as is done with the transfixation cast. After the cast hardens completely, the caudal quarter to third of the cast can be removed and the padding cut away from the caudal aspect of the limb. This modification allows unencumbered movement of the gastrocnemius. Occasionally the dorsal distal portion of the cast also must be trimmed to allow flexion of the hock. Some patients initially require a splint or bandage over the fetlock to ensure the animal bears weight on the solar surface of the foot. Most patients become fully ambulatory in 48 to 72 hours. Treatment of young animals should be tailored to prevent a compensatory tarsal varus of the contralateral limb. This procedure is technically less difficult in that it allows more variation in pin placement than if traditional connecting bars are used. The pin ends should be covered as they are in transfixation casting.30
Splints
Splints can be useful in treating some musculoskeletal conditions in small ruminants. However, the veterinarian should be selective in using them. Many practitioners are more comfortable using casts and external fixators than applying and monitoring splints. Many of the small ruminants presented to referral centers for malunion or delayed union of fractures have previously been treated with splints. For this reason alone, the initial use if other techniques that achieve more stable fracture fixation should be considered. However, splints can be useful in selected cases if the practitioner is skilled at splint management. In emergency situations, a splint can be made of cut polyvinyl chloride (PVC) pipe or other such material.27
A spoon splint, either commercially manufactured or fashioned from cast material, probably is best used to support greenstick fractures of the distal limb. When used in this way, the spoon splint helps prevent catastrophic breakdown of the fracture. However, a more important role may be in preventing the limb contracture that can occur if the carpus is allowed to remain flexed for a prolonged period in a non–weight-bearing animal. With this technique, a padded bandage is placed on the limb and the splint is conformed to the bandage and secured with adhesive tape.
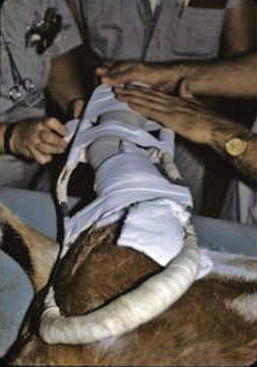
Another type of splint occasionally used in small ruminants is the traction splint, commonly referred to as the Schroeder-Thomas splint (Figure 11-5). This splint usually is made of aluminum rods and consists of a ring that fits in the axillary or inguinal region of the animal with bars on the dorsal and palmar or plantar aspect of the limb joined distally. The shape of the splint varies, as does the way particular parts of the limb are secured to the splint depending on the specific reason the splint is applied. Traction is applied by securing the foot to the distal splint with adhesive tape or by placing wires through the hoof wall. A soft bandage should be placed on the limb, after which the limb is secured strategically to the splint. Usually tape is placed over the entire limb and distal splint.11
1. US Department of Agriculture. Sheep and lamb predator death loss in the United States, 2004. Fort Collins, Colo: USDA: APHIS:VS:CEAH, National Animal Health Monitoring System; 2007.
2. Kaneps A.J. Orthopedic conditions of small ruminants. Vet Clin North Am Adv Rumin Orthop. 1996;12:211.
3. Nyack B., Padmore C.L., White M. External fixation of carpal and metacarpal fractures in a goat. Bovine Pract. 1982;3:23.
4. Smith MC: Practice tips for small ruminant veterinarians. Proceedings of the 69th annual Western Veterinary Conference, Las Vegas, Nev, 1998.
5. Mbiuki S.M., Byagagaire S.D. Full limb casting: a treatment for tibial fractures in calves and goats. Vet Med. 1984;79:243.
6. Monin T. Tension band repair of equine mandibular fractures. J Eq Med Surg. 1977;1:325.
7. DeBowes R.M. Equine fracture repair. Philadelphia: WB Saunders; 1996.
8. Nunamaker D.M., et al. A new skeletal fixation device that allows immediate full weight bearing application in the horse. Vet Surg. 1986;15:345.
9. Egger E.L., Greenwood K.M. Textbook of small animal surgery. Philadelphia: WB Saunders; 1985.
10. St-Jean G., Clem M.F., DeBowes R.M. Transfixation pinning and casting of tibial fractures in calves: five cases (1985-1989). J Am Vet Med Assoc. 1991;198:139.
11. Arnoczky S.P., Blass C.E., McCoy L. External coaptation and bandaging. In: Slatter D.H., editor. Textbook of small animal surgery. Philadelphia: WB Saunders, 1985.
Infectious Conditions
Septic Arthritis
Bacterial infections of the joints (septic arthritis) occur most commonly in neonates. However, older sheep and goats sporadically suffer from joint infection as a result of a penetrating injury or spread from adjacent infected tissues, as in the case of footrot. In neonates, septic arthritis is most often a sequela of septicemia and often is a consequence of failure of passive transfer.1 Bacteria isolated from lambs include Streptococcus, Escherichia coli, Arcanobacterium pyogenes (formerly Actinomyces pyogenes), Erysipelothrix insidiosa (rhusiopathiae), Pasteurella haemolytica, Corynebacterium pseudotuberculosis, and Fusobacterium necrophorum. Staphylococcus aureus arthritis is associated with tick pyemia, a disease seen in lambs 2 to 6 weeks old in areas infested with Ixodes ricinus ticks. Streptococcus dysgalactiae has been reported as a cause of arthritis in dairy goats and was the most common pathogen isolated from arthritic lambs in England and Wales. Other isolates included E. coli, coagulase-positive Staphylococcus, E. rhusiopathiae, and A. pyogenes.2 Coexisting omphalitis was found in 16% of arthritic lambs.
Erysipelothrix polyarthritis is a nonsuppurative condition usually seen in 2- to 6-month-old lambs, but it also may be seen in neonates. Outbreaks may affect as many as 40% of the lambs in a flock. Hallmarks of this infection are fever and lameness, with minimal swelling of joints. This nonsuppurative polyarthritis will progress to chronic arthritis if not treated appropriately.1
Diagnosis
A sterile aspirate of synovial fluid should be obtained and the fluid submitted for culture and cytology. The character of the synovial fluid varies according to the etiology and stage of disease. Synovial fluid from infected joints may be thin and watery (lacking normal viscosity) or thick and cloudy with purulent material. Infected synovial fluid often demonstrates characteristic pleocytosis and neutrophilia (more than 30,000 to 100,000 white blood cells/μL and more than 75% neutrophils), as well as an increased total protein (see Appendix 2, Table 2-9). Not all aspirates from septic joints yield bacteria, but some do. Culture results may yield more definitive results with the use of enhancement media or synovial membrane biopsy, particularly if the animal has previously been treated with antimicrobial agents. Radiography may be used to determine the severity of degenerative changes, although bone changes may not be visible for several days after the onset of disease. Radiographic evaluation may be more important to monitor the progression of septic arthritis during therapy. Ultrasonography also may be useful in evaluating existing soft tissue pathology.
Treatment
The administration of antimicrobial agents and joint lavage are the mainstays of treatment of septic arthritis. Antimicrobials, which may be administered systemically or intraarticularly, should be chosen on the basis of an assessment for specific pathogens (gram-positive bacteria are more likely) and culture results when available.3
Regional limb perfusion with antibiotics is an adjunctive procedure that may be beneficial in some cases.3 This technique entails instilling small volumes of antimicrobial agents in targeted locations to achieve high concentrations in infected areas. Regional perfusion can be accomplished with intramedullary administration of antimicrobial agents but is more easily and commonly performed by intravenous injection distal to a tourniquet. Sheep and goats generally should be sedated before this procedure. The skin over the peripheral vein is aseptically prepared. The clinician inserts a needle (20- or 21-gauge) into the vein in a proximal direction and infuses the antibiotic of choice (ceftiofur sodium, 1 mg/kg, or potassium or sodium penicillin, 20,000 IU/kg). For repeated administration in chronic conditions, a catheter (22-gauge) can be placed in the vein and the leg wrapped to help maintain catheter patency.4 The prognosis with septic arthritis is guarded, and chronic lameness is a sequela in many cases.
Prevention
Ensuring adequate passive transfer in neonates helps prevent septicemia and septic arthritis resulting from hematogenous spread of bacteria to joints. Maintaining a clean environment for lambing and kidding and providing appropriate umbilical care also will help prevent neonatal septicemia.
Chlamydial Polyarthritis
Chlamydial polyarthritis is a common contagious disease of feedlot lambs in the United States. The disease is suspected to occur in goats as well.5 The causative agent formerly was considered to be a strain (immunotype 2) of Chlamydia psittaci but has been reclassified as Chlamydophila pecorum.6,7 Economic losses associated with chlamydial arthritis result from weight loss and treatment costs. Disease occurs in 1- to 8-month-old lambs, with 3- to 5-month-old lambs most commonly affected.8 Outbreaks in feedlots often occur a few weeks after lambs are introduced.8 Morbidity can be as high as 80%, with less than 1% mortality.7
Pathogenesis
C. pecorum organisms are present in nasal and ocular secretions, feces, and urine of infected animals.8 As many as half the lambs on some farms shed C. pecorum in feces without signs of clinical disease.6
Clinical Signs
Affected lambs have fever (with temperatures up to 108° F) and are reluctant to move, often appearing “tucked up” or becoming recumbent. Lameness is apparent in one or more limbs, and affected joints typically are enlarged.5,8 Chlamydial conjunctivitis may occur concurrently.8–10 The course of the disease is approximately 10 to 14 days without treatment. Most lambs recover, but some remain lame.5 Significant necropsy findings include fibrinous exudate in joints and edema of surrounding tissue. The articular cartilage is minimally affected.5,10
Diagnosis
Joint fluid may contain fibrin but is not purulent. Elementary inclusion bodies may be seen on Giemsa-stained smears of synovial fluid. Isolation of Chlamydia requires special media and is not routinely performed. The use of DNA-based tests should aid and improve the understanding of the epidemiology of different chlamydial infections.11
Treatment and Prevention
Oxytetracycline (20 mg/kg given subcutaneously [SC] or IM every 48 to 72 hours), erythromycin (3 to 5 mg/kg IM three times a day [or twice daily]), and tylosin (20 mg/kg IM twice a day) may be useful.7 Treatment early in the course of disease speeds recovery.5,10 During an outbreak, lame and febrile lambs should be isolated from healthy lambs to minimize the spread of infection. A vaccine is available for chlamydial abortion, but researchers have not determined whether it provides protection against C. pecorum arthritis (see Chapter 8).
Mycoplasmal Polyarthritis
Mycoplasmal arthritis is a highly fatal disease of goats marked by polyarthritis, septicemia, and mastitis. This disease usually is caused by Mycoplasma mycoides subsp. mycoides Large Colony (MmmLC), recently reclassified as a serovar of Mycoplasma mycoides subsp. capri.12 Other mycoplasmal species (Mycoplasma agalactiae, Mycoplasma capricolum, Mycoplasma putrefaciens) cause similar syndromes.13 This is distinct from the small colony (SC) or bovine biotype of Mmm that causes contagious bovine pleuropneumonia (CBPP), a disease eradicated from the United States in 1892. Sheep may be experimentally infected, and natural infection in sheep is suspected to occur.14
Mycoplasmal arthritis occurs as an epizootic condition in many countries throughout the world. In the United States, most outbreaks are in large goat dairies. Morbidity and mortality rates as high as 90% have been reported in kids.15 M. putrefaciens was responsible for the loss of 700 goats in one California dairy.16
Mmm usually is introduced to a farm by an asymptomatic shedder. The bacteria are shed in the colostrum and milk of infected does, and ingestion is thought to be the primary source of infection of kids.14–16 In one outbreak, approximately half of the does were noted to shed Mmm organisms in milk. Some were intermittent asymptomatic shedders, but clinical mastitis ultimately developed in most animals.17 Horizontal transmission was documented among kids housed together and is likely to occur among adults, especially in the milking parlor.18 Illness often follows stress such as from castration, dehorning, concurrent disease, bad weather, and overcrowding.16,17,19
Pathogenesis
Infection leads to mycoplasmosis with involvement of numerous body systems; fibrinous polyarthritis, pneumonia, peritonitis, mastitis, conjunctivitis, and pericarditis are among the more common presentations. If animals recover, the organisms may be shed in ocular and nasal secretions and in milk.20
Clinical Signs
Kids 3 to 8 weeks old are most susceptible, but animals of any age may be affected. Clinical signs include fever, warm swellings of numerous joints, mastitis, lameness, conjunctivitis, weight loss, and pneumonia. Three syndromes have been described in kids. A peracute form results in death in 12 to 24 hours with fever being the only sign. A second group of kids showed signs of brain disease (opisthotonos) and died in 24 to 72 hours. The third syndrome was characterized by fever, warm swollen joints, lameness, recumbency, and pneumonia. Many in this group died within a few days, but some lame kids recovered over a few weeks.15 Adult females may develop acute or peracute mastitis, the latter causing death in 1 to 3 days. Does that recover may have udder fibrosis and may shed Mmm organisms intermittently. Arthritis is a less common finding in adults than in kids. Mastitis and severe lameness without fever were observed in an M. putrefaciens outbreak.16
Diagnosis
Postmortem findings include suppurative polyarthritis, osteomyelitis, fibrinous pleuritis, pneumonia, peritonitis, meningoencephalitis, and pericarditis.15,17,19 The joints most commonly affected are the carpus, stifle, tarsus, hip, and elbow. Joint fluid is purulent and contains fibrin, and the joint capsules are thickened, with erosions of articular cartilage. Mmm can be cultured from synovial fluid and from many internal sites.17
Treatment
Antibiotic treatment does not eliminate infection in most cases. Some animals appear to improve, only to relapse later. Tylosin is the antibiotic most commonly recommended (10 to 50 mg/kg three times a day), but its efficacy is uncertain.20 Antimicrobial susceptibility may vary with strain, but an in vitro study suggests that tylosin, erythromycin, oxytetracycline, or enrofloxacin may be effective. This application would be an extralabel use of enrofloxacin, which is prohibited by the U.S. Food and Drug Administration (FDA).21
Prevention
Effective preventive measures for kids include the feeding of heat-treated colostrum and pasteurized goat milk. Disease in adults can be controlled by identifying carriers by milk culture and either culling carriers or isolating infected animals and milking them after uninfected animals. Culture of milk samples from individual does and the bulk tank should be performed periodically to identify newly infected animals or intermittent shedders, and colostrum should be cultured at the time of freshening. No vaccine is currently commercially available (see Chapter 15).
Osteomyelitis
Bone infections usually result from hematogenous spread of organisms or from direct inoculation associated with trauma to soft tissues covering the bone. The soft tissue damage may be from either an acute injury (trauma or surgical incision) or that associated with development of decubitus ulcers in a recumbent animal. Occasionally the tissue damage is incurred during normal recumbency when animals are housed on hard, rough surfaces and is not a sequela of debilitation. The infectious agents include Corynebacterium, A. pyogenes, Rhodococcus equi,22 and E. coli.
Clinical Signs
Lameness, pain on palpation, and focal swelling are common clinical signs of osteomyelitis. Severe lameness may result in recumbency. Infection of vertebrae may produce signs of spinal cord dysfunction.7
Treatment and Prevention
The prognosis is guarded. Antimicrobial therapy alone is rarely successful because of its poor penetration of infected bone. Surgical débridement of infected tissue is an important component of therapy. Antibiotics, particularly those used based on culture and sensitivity patterns, should be continued for several weeks after surgical débridement. Regional perfusion of antibiotics may be useful in treating osteomyelitis. Amputation is the only possible way to rid the animal of infection in some cases. The possibility of control of infection varies with the cause of the infection. Environmental control probably is the most important mechanism to prevent trauma to the animal. Adherence to aseptic technique when performing any surgery on or near osseous structures decreases surgical infection.
Caprine Arthritis-Encephalitis
Caprine arthritis-encephalitis (CAE) is a chronic multisystemic disease of goats caused by a nononcogenic retrovirus. Infection with caprine arthritis-encephalitis virus (CAEV) is widespread, and chronic polyarthritis is the most common clinical manifestation.23 CAEV is closely related to the viruses that cause ovine progressive pneumonia (OPP) and maedi-visna,24 and together these are referred to as small ruminant lentiviruses (SRLVs). Phylogenetic analysis has determined four sequence groups, designated A to D, and several subtypes for SRLVs. Some subtypes of these viruses occur in both sheep and goats, and there is evidence of transmission of SRLVs between the species.25
Seroprevalence rates for CAEV in goats in the United States, Canada, and Europe range from 38% to 81%.23,26,27 Seroprevalence in England, Australia, and developing countries usually is less than 10%.28 Clinical arthritis is estimated to occur in less than 25% of seropositive animals but it may be more prevalent in some herds.23,27 The prevalence of other clinical syndromes is not known. Infection occurs by transmission of fluids that contain infected macrophages from an infected animal to an uninfected animal. The most efficient manner of transmission is from dam to kid by ingestion of colostrum or milk from infected does.29 The presence of antiviral antibodies in colostrum is not protective. Feeding nonpasteurized milk increases the risk of infection.26,27
Horizontal transmission of CAEV also is important.29,30,31 When uninfected goats are housed with infected goats for long periods, a significant number seroconvert.29 Uninfected does readily seroconvert when milked with infected does, presumably as a result of transfer of the virus during the milking process.29 Venereal transmission is possible, especially if one of the animals exhibits clinical disease.32 Transmission from doe to kid before or during parturition has been documented.30 No evidence supports transmission by an insect vector. Iatrogenic transmission (on dehorning equipment or needles) also is possible. The likelihood of transmission from a contaminated environment is very low.31,32
Pathogenesis
The important target tissues of CAEV include the joints, mammary glands, lungs, and brain. At these target sites, CAEV induces chronic inflammation by invoking the host’s immune responses. The virus is capable of making antigenic variants to help it evade the host immune response. CAEV often can be isolated from the synovial fluid and milk of infected animals.23,29 Disease results from inflammation elicited by the reaction of the immune system to the virus. Infected macrophages express viral proteins near major histocompatibility complex (MHC) antigens, which are recognized by T lymphocytes and stimulate cytokine production. Goats usually seroconvert in 2 to 8 weeks, but a long clinical latency (spanning years) is possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree