Chapter 15 Diseases of the Mammary Gland
Normal Anatomy of the Mammary Gland
The mammary structure of the sheep and goat consists of two functionally and anatomically separate glands (halves), each with one teat. Each half is supported by a medial and a lateral suspensory ligament; in turn, these ligaments branch off as secondary laminae that enter into and support the gland tissue. The medial suspensory ligaments are adhered together and run on midline from the prepubic symphysial tendon to the abdominal tunic. The intramammary groove is formed where the medial suspensory ligaments meet the skin of the ventral udder. The elastic medial ligament should hold the udder high and tight to the abdominal wall above the level of the hocks; heritability of the medial suspensory ligament conformation is 0.33,1 and breeding programs should specifically select against a pendulous udder. The lateral suspensory ligaments run deep to the skin and superficial to the mammary neurovascular and lymphatic structures. The draining supramammary (superficial inguinal) lymph node is located at the dorsocaudal aspect of each gland. The main arterial supply to the udder is from the external pudendal arteries, which emerge from the inguinal rings and can be readily identified by their tortuous path. The paired external pudendal veins, paired branching subcutaneous abdominal veins, and paired perineal veins drain the udder and the supramammary lymph nodes. The gland is innervated by the genitofemoral nerve with superficial contributions from the lumbar cutaneous nerves (cranially) and from the mammary branch of the pudendal nerve (caudally).
Each half of the udder consists of multiple gland lobes that drain into six to nine milk ducts.2 These ducts coalesce to form a gland cistern, which in turn drains into the teat cistern. Furstenberg’s ring, an annular venous structure, forms the demarcation between udder and teat. As in cattle, the teat wall consists of five layers: mucosa, vascular connective tissue, circular and longitudinal muscular layers, and epithelium. A 0.5- to 1.0-cm streak canal at the distal end of the teat connects the teat cistern to the teat orifice and is identified proximately by the rosette of Furstenberg. The streak canal is an important anatomic and physiologic barrier to the udder with its keratin-producing squamous epithelial cell lining and muscular teat sphincter.
Production and Component Benchmarks
Species and operation type significantly influence various aspects of lactation. Dairy goats typically are milked for a 305-day lactation with a 60-day dry period, in a regimen similar to that for dairy cattle. It is common for kids to be hand-reared on pasteurized milk or milk replacer. Dairy sheep have a much steeper lactation curve of approximately 5 months, similar to that for sheep and goats in meat and fiber operations. A common practice in dairy sheep operations is to dam-raise the lambs for the first 1 to 2 months and then switch over to machine milking for commercial milk production, the suckling and suckling-to-milking periods offer some unique challenges with regard to mastitis control. Nutrition, mastitis, and reproduction are the major factors influencing production in all herd types (see Chapters 2 and 8).
Milk volume and composition differ among the dairy species. Compared with milk from goats and cows, sheep milk is highest in fat (7.62%), proteins (6.21%), caseins, and other solids.3 As a result, rennet coagulation time is shortened and curd firmness is improved during cheese production. However, dairy sheep produce less milk volume per lactation period, and genetic potential differs significantly between European and U.S. lines. As a rough average, the East Fresian breed produces approximately 1000 lb of milk per lactation, and the Lacaune lait breed, evaluated on an as-milked basis (excluding milk used to dam-rear the lambs), produces around 650 lb per lactation.
Goat milk frequently is prescribed for milk-intolerant children and adults; 40% of people who cannot digest cow milk will be able to tolerate goat milk.3 Digestibility is facilitated by smaller fat globules and a higher proportion of short-chain fatty acids, such as the appropriately named caproic, caprylic, and capric acids.3 Dairy goats produce more milk volume than that typical for sheep, with component percentages falling between those of cattle and sheep; production volume and components are significantly influenced by breed, herd, and individual genetic potential. Miniature breeds produce a smaller volume of milk with higher fat and protein content. The average milk production in Wisconsin herds for 2008 was 1288 lb/doe, with the top four herds averaging 1510 lb/doe.4 By comparison, the 2009 “honor roll” members of the California Dairy Herd Improvement Association (DHIA) produced as much as 2717 and 603 lb/doe for standard and miniature breed dairy goats, respectively.5
In the standard European breeds, a 3.8% fat content is typical.3 Among the top California operations, fat contents as high as 4.9% and 6.9% were recorded for standard and miniature breeds.5 In one high-volume operation, however, the result of a butterfat test was very low at 2.7%. As in cow milk, milkfat in goat milk can be suppressed by highly fermentable diets (with a carbohydrate-to-forage ratio greater than 2:1), subacute ruminal acidosis, and heat stress. Increasing dietary forage and offering free-choice buffers (e.g., bicarbonate) will help raise measured butterfat content. In the standard European breeds, protein averages 2.9%.3 Among the top California operations protein ranged from 2.61% to 3.96% and 3.91% to 5.0% in the standard and miniature breeds.5
Somatic Cells
In small ruminants, increased somatic cell counts (SCCs) are associated with increased parity, days in milk, stressors, and onset of estrus, as well as with infection. The contribution of these factors is compounded in a seasonally producing herd. In a survey of 71 U.S. goat dairies, 65% did not meet grade A standards of 1 million cells/mL near the end of their lactation cycle,6 and many had difficulty meeting grade B standards of 1.5 million cells/mL. Apocrine milk production in small ruminants complicates SCC determination because some testing methods will miscount normal DNA-free cytoplasmic droplets; goats produce 10 times more cytoplasmic droplets than sheep.7 The Levowitz-Weber stain used for cattle SCC determinations does not adequately differentiate between leukocytes and cytoplasmic droplets.7 Direct microscopic counts using the pyronin Y methyl green stain is specified by the U.S. reference standard for small ruminant milk, but staining methods and technician competency may vary by laboratory.7,8 With Fossomatic techniques, counts are in good agreement with the reference standard.6,8
Normal somatic cell populations differ dramatically between the species. Neutrophils are the most common leukocyte in both the infected and uninfected caprine mammary gland, making up 74% to 80% of the cell population in late lactation.9 By comparison, the noninfected ovine mammary gland cell population is comparable to that in cattle, being largely composed of macrophages (45% to 85%), with fewer neutrophils (10% to 35%), lymphocytes (10% to 17%), and epithelial cells (2% to 3%); neutrophil numbers increase during infection and are highly correlated with SCC.7,9
The degree to which infection directly correlates with SCC is controversial. Some workers suggest that IMI status is the major variable factoring into SCC.9,10 In one goat dairy, however, although SCC increased with IMI prevalence, 90% of SCC variability relates to factors other than mastitis.11 In both species, higher SCCs in early and midlactation are more likely to indicate infection than equivalent counts in late lactation,2,9 and repeated tests, or comparative samples between udder halves, are more informative than single test points.9 SCC also is moderately heritable, estimated at approximately 0.11 to 0.15 in the larger sheep breed databases. French Lacaune breeders are trying to reduce SCC through selective breeding.9
Bacterial Pathogens
Bacterial pathogens responsible for clinical and subclinical mastitis in small ruminants are well characterized. Sporadic cases of clinical mastitis most frequently are caused by Staphylococcus aureus, coagulase-negative Staphylococcus spp., Arcanobacterium pyogenes, Corynebacterium, Pasteurella spp., and Pseudomonas spp.9 Outbreaks of clinical mastitis most frequently involve S. aureus, Streptococcus spp. (S. uberis, S. agalactiae, and S. suis), and opportunists such as Aspergillus, Pseudomonas, Burkholderia, and Serratia.9
Numerous studies have identified coagulase-negative Staphylococcus spp. as by far the most important cause of subclinical mastitis in both the ewe (78%) and doe (71%). S. epidermidis and S. caprae are isolated most frequently, although other species are commonly identified.8,9 Shedding of coagulase-negative staphylococci often is cyclic, in inverse proportion to SCC elevation, and may be missed on single culture. From 60% to 80% of cultured strains of coagulase-negative staphylococci are hemolytic; hemolytic strains, and S. epidermidis as a species, tend to cause very high elevations in SCC, whereas other coagulase-negative staphylococcal species may not be obviously associated with an elevated SCC.9 S. aureus is the second most frequently isolated subclinical mastitis agent in the ewe (4%) and doe (8%), whereas Streptococcus spp. and Corynebacterium are less frequently identified.9 Unlike in dairy cattle, gram-negative bacteria are infrequent causes of mastitis in the ewe (3%) and doe (8%).9 Although rarely involved in mastitis, Listeria and Salmonella spp. are worth mentioning owing to their zoonotic potential; Listeria can be shed from clinically normal udders.2
Functional Abnormalities and Therapies
Congenital Abnormalities
Supernumerary Teats
The normal conformation of the udder in both sheep and goats includes the presence of two teats, one on each half of the udder; however, some animals may be identified with three to six teats. Dairy breed organizations (for both goats and sheep) often identify supernumerary teats as a serious disqualification in both sexes and prohibit the surgical removal and subsequent registration of purebred animals with extra teats. In meat animals, less emphasis is placed on teat conformation, and many meat breed animals have supernumerary teats. In some instances, breeders have even advocated the selection of meat breed replacement animals that have four “clean” teats (i.e., fully and separately developed) as a means of increasing productivity.12
Although detailed studies of the inheritance of supernumerary teats in small ruminants are not available, a genetic mode of inheritance has been recognized. Consequently, attention to the teat structure in breeding males and females should be of high priority, and animals with unacceptable teat conformation should be culled. Surgical correction of supernumerary teats, especially those classified as a disqualification, does not address the genetic inheritance of this condition and only prolongs and increases the prevalence of this defect in the breeding population.
Weeping Teats and Teat Wall Cyst
In some animals selected for high milk production, milk-secreting tissue may be present in the wall of the teat. Three outcomes are possible relative to the milk produced by such tissue: (1) In some instances, the milk passes through local pores into the teat cistern, with no clinical evidence of presence of this tissue. (2) Alternatively, the milk can pass through skin pores in the external epithelial surface of the teat and be released onto the skin surface, resulting in a “weeping teat.” Because the muscular orifice typical of the teat streak canal is absent, this tissue may be prone to development of retrograde bacterial infections and localized mastitis. Clinically, animals with weeping teats are easily identified by the presence of milk on the lateral external surface, particularly at the time of milking. Owners of affected animals also may report that during hand-milking, their hands become wet with milk. Apart from the aesthetic downside of these lesions and the very occasional associated mastitis, they generally do not pose significant health problems for affected animals. The use of silver nitrate sticks to cauterize these weeping pores has been reported2; however, this procedure may potentially lead to the development of a teat cyst, as described further on. (3) Finally, if no porous passage exists for the milk to move out of the teat wall, a teat wall cyst will develop to contain the accumulating milk. In such cases, the cyst can be readily identified clinically by detection of a focal fluctuant swelling in the teat wall. Teat wall cysts may be as small as a couple of millimeters in diameter up to 1 to 2 cm in diameter. Ultrasonographic evaluation of the teat (as just described for bifid teats) will readily identify a hypoechoic fluid-filled structure located in the teat wall. Aspiration of the cyst, performed using aseptic technique, will confirm the diagnosis.2 In some instances, presence of the cyst may lead to difficulty in placing the teat cup on the teat; however, this problem generally is of limited importance. Perhaps more significant is the occasional teat cyst that results in deformation of the mucosal wall of the teat cistern, with consequent functional outflow obstruction of milk through the teat canal. In such cases, ultrasonography-guided aspiration of the cyst may restore milk flow, and surgical resection of the teat cyst can be performed if warranted.
Uneven or Asymmetric Udder
Asymmetric udders or uneven udders can occur as both a congenital and an acquired condition. In rare instances, the suspensory ligaments of the udder are attached in an asymmetric fashion, which results in a “twisted” appearance of the udder in relation to the main body axis. In dairy goats, it is relatively common for does to have an asymmetric udder associated with uneven milk production. This situation may be present from the time of parturition or may develop over the course of the lactation period. In some cases this finding may be associated with a subclinical infection with coagulase-negative staphylococci on the side with less milk production, so milk culture of each half performed separately is suggested. If the condition occurs as a herd-level problem, a thorough evaluation of the milking system should be conducted in addition to individual animal milk cultures. Milking system cleaning and disinfection practices should be evaluated, as well as milking claw design and placement during milking. In our own practice, we have observed a herd-level problem with asymmetric udders associated with placement of milking claws from the side, resulting in differential milking rates from the halves and, consequently, differential milk production.
Physiologic Abnormalities
Udder Edema
Udder edema is a common finding in recently fresh animals, especially primiparous does and ewes. Careful evaluation of an enlarged mammary gland is indicated to differentiate between mastitis and edema. In mastitis, the gland often will be enlarged, may be either very warm or very cold to the touch, and may be painful and typically expresses milk with an abnormal-looking texture or color or with an odor. In edema of other causes, the clinical presentation also will include mammary gland swelling but the milk will be normal. Considerations in the differential diagnosis for udder edema should include trauma, hypoproteinemia, recent parturition (fresh doe or ewe), and dependent edema. A less obvious but nonetheless important possibility is hypoproteinemia associated with intestinal parasitism. In one herd, udder edema was the first clinical sign of hypoproteinemia and resolved after appropriate therapy for the parasitic infection (see Chapter 6).
Gynecomastia
Gynecomastia refers to the abnormal development of a mammary system and milk secretion in a male. Three different causes have been identified in small ruminants, particularly goats. In two published reports, the animals had evidence of sex chromosome abnormalities, one with Y chromosome deletions and the other with sex chromatin in the neutrophils.13,14 Gynecomastia also has been reported to occur as the consequence of a familial predisposition associated with high milk production in the maternal line. It is speculated that the affected animals may have higher baseline production of prolactogenic hormones that lead to the abnormal mammary gland development.15 Similarly, animals with endocrine imbalances associated with adrenal tumors may exhibit gynecomastia.16 Finally, excessive mechanical stimulation of the teats associated with simulated milking or nursing appears to be sufficient to elicit mammary gland development with secretion of small volumes of milk.15
In many cases of short-term gynecomastia, the fertility of the buck may not be affected; nevertheless, a full breeding soundness exam is always warranted. When the mammary gland is excessively large, it may interfere with normal cooling of the testicles, with the potential for decrease in or loss of fertility.15 With abnormalities involving the sex chromosomes, the affected animal generally is infertile.
Obstructions to Flow
Teat Spider and Lactoliths
In cases of blockage palpation of the teat often will reveal a firm pea-size mass that may be movable in the teat cistern. Ultrasound examination can be performed as described in the section “Diagnostic and Therapeutic Procedures” and can reveal the presence of a tissue mass extending from the mucosa surface of the teat cistern. With this type of lesion, two basic forms of therapy have been used: Various forms of teat knives can be introduced through the streak canal and used to macerate the teat spider so that it can be removed in smaller portions.17 Alternatively, a surgical thelotomy may be performed to remove the mass. Anesthetic block is obtained with local infiltration of lidocaine in a circumferential pattern at the base of the teat. For the procedure, a 3- to 4-cm-long incision is made parallel to the length of the teat. A teat cannula should be passed through the streak canal and used to protect the mucosa of the opposite side of the teat cistern during entry. The mucosa surrounding the lesion should be undermined and its edges apposed with monofilament suture17 to prevent excessive granulation tissue from developing and occluding the teat cistern. The submucosa and intermediate layer are closed in a continuous horizontal pattern using resorbable monofilament suture, and the skin is closed with simple interrupted sutures.
Common Surgeries of the Teat and Udder
Teat Laceration Repair
Preoperatively the animal can be sedated if necessary, and a ring block with 2% lidocaine is performed around the base of the teat, with care taken to avoid the circumferential vein and the teat and gland cistern. If the laceration is full-thickness, some clinicians also place a tourniquet at the base of the teat to minimize interference with surgical visualization by milk from the teat. If necessary, the wound should be surgically debrided, with preservation of as much tissue as possible. Full-thickness lacerations should be closed in three layers.17 First the submucosa is closed using a continuous horizontal pattern that does not penetrate the mucosa, followed by closure of the intermediate layer using a similar pattern, best accomplished with 4-0 monofilament synthetic resorbable suture introduced by a swaged-on taper needle. Finally the skin is closed using 4-0 or 3-0 monofilament suture in a simple interrupted pattern. Postoperatively, the patient should not be subjected to mechanical milking or hand-milking for at least 10 days. Instead, the milk should be passively removed from the teat cistern using a teat cannula. Intramammary antibiotics should be given every other day during this time, and the mammary gland should be closely monitored for signs of mastitis.
Mastectomy
Radical mastectomy is a treatment for mammary conditions such as gangrenous mastitis not responsive to medical treatment, precocious udder that exhibits inappropriate lactation, or other localized mammary disease. Goats with gangrenous mastitis present with clinical signs of a discolored (dark) udder that is cold, painful, and swollen. The milk usually is blood-tinged. Most animals are affected at 10 to 15 days after kidding. Medical treatment is seldom successful, and chronic mastitis frequently is the end result.18 Mastectomy has proved to be a safe and effective treatment to allow good quality of life in pet animals or in genetically valuable animals to be used as embryo donors, or in natural dams of offspring to be hand-raised.19
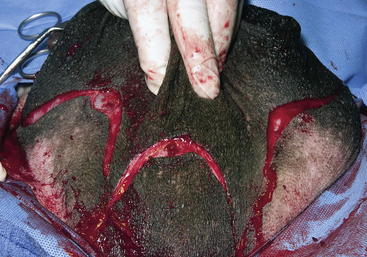
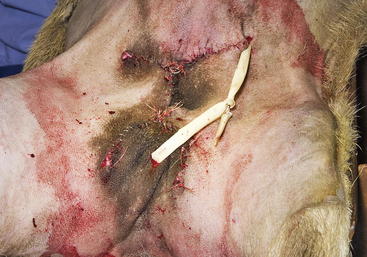
A radical mastectomy is performed with the animal in dorsal recumbency under general anesthesia (Figure 15-1). This positioning allows access to more skin for closure with minimal tension. Some veterinary surgeons prefer an elliptical skin incision. The inverted cloverleaf skin incision, however, allows dissection of the skin away from the mammary tissue and identification of the vasculature to allow ligation of the vessels to prevent hemorrhage (Figure 15-2). The arterial blood supply to the mammary gland arises from the external pudendal and perineal arteries. The blood drains from the gland by way of the external pudendal and perineal veins as well as the large subcutaneous abdominal vein. The mammary tissue can be bluntly dissected off the external rectus sheath by fanning of the operator’s hand under the glandular tissue. The skin closure is then done in an X shape, with latex drains placed subcutaneously exiting away from the incision line (Figure 15-3). The dissection leaves abundant dead space, which should be ablated as much as possible by tacking the subcutaneous tissue to the external rectus sheath with absorbable sutures.
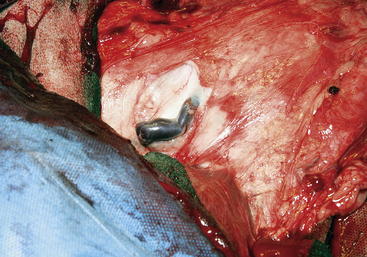
Figure 15-2 The external pudendal vein near the right inguinal ring, in the doe shown in Figure 15-1. In this caudal view, the skin has been dissected to the left and the mammary tissue is to the right.
(Courtesy Dr. A.N. Baird, Purdue University.)
Partial mastectomy may be performed in the case of unilateral disease. The partial mastectomy is done through an elliptical incision around the teat of the affected gland. Partial mastectomy is technically more difficult to perform because of collateral circulation and different dissection required. Care must be taken in the dissection not to compromise the gland to be left intact.20
An alternative to radical mastectomy in does with gangrenous mastitis is ligation of the mammary vasculature in conjunction with the amputation of the teat. This surgical approach allows drainage of the glandular discharge and ultimately avascular necrosis of the udder. When compared with a traditional radical mastectomy, this method was described as quicker to perform, less expensive, and less stressful to the goat.21 However, the sloughing udder may not be cosmetically pleasing to the owner.
Ligation of the External Pudendal Artery
The patient with severe gangrenous mastitis may be an unsatisfactory anesthetic risk. In such cases, one option is to ligate the external pudendal artery and vein as they exit the inguinal canal. This procedure can be performed with use of mild sedation and local anesthesia with the animal in lateral recumbency. After ligation of these vessels, the absorption of toxins from the mammary gland is limited, and the mammary gland will atrophy because the external pudendal artery is the primary vascular supply for the mammary gland of sheep and goats.17
For this procedure, the animal is placed in lateral recumbency, and the area external to the inguinal canal is infiltrated with 2% lidocaine. A skin incision is made over the region, and blunt dissection is used to identify the inguinal canal with both the external pudendal artery and vein exiting it. Each vessel is triple-ligated and incised, leaving two ligatures on the cardiac side. The dead space is minimized using several layers of subcutaneous sutures and the skin is closed in a routine fashion. The clinician also may consider teat amputation after this surgery, in order to facilitate drainage of the mammary gland.17
1. Wiggans G.R., Hubbard S.M. Genetic evaluation of yield and type traits of dairy goats in the United States. J Dairy Sci. 2001;84(Suppl 1):E69-E73.
2. Smith M., Sherman D. Mammary system. In: Smith M., Sherman D., editors. Goat medicine. Ames, Iowa: Wiley-Blackwell, 2009.
3. Jandal J.M. Comparative aspects of goat and sheep milk. Small Rumin Res. 1996;22:177-185.
4. Dietman P., Tranel L. The Wisconsin goat dairy profitability project: 2007 and 2008 results for a select group of Wisconsin goat dairies. Madison, Wisc: Wisconsin Department of Agriculture, Trade and Consumer Protection; 2009. A collaborative project of the Wisconsin Department of Agriculture, Trade and Consumer Protection, Wisconsin Technical College System, University of Wisconsin-Extension, Iowa State University Extension, and Southwest Badger Resource Conservation and Development Council
5. California Dairy Herd Improvement Association: 2009 honor roll (website): http://caldairygoats.com/cdhiagoats.htm. Accessed February 1, 2011.
6. Droke E.A., Paape M.J., Di Carlo A.L. Prevalence of high somatic cell counts in bulk tank goat milk. J Dairy Sci. 1993;76:1035-1039.
7. Paape M.J., et al. Milk somatic cells and lactation in small ruminants. J Dairy Sci. 2001;84(Suppl 1):E237-E244.
8. Contreras A., et al. Mastitis in small ruminants. Small Rumin Res. 2007;68:145-153.
9. Bergonier D., et al. Mastitis of dairy small ruminants. Vet Res. 2003;34:689-716.
10. Poutrel B., et al. Control of intramammary infections in goats: impact on somatic cell counts. J Anim Sci. 1997;75:566-570.
11. Wilson D.J., Stewart K.N., Sears P.M. Effects of stage of lactation, production, parity and season on somatic cell counts in infected and uninfected dairy goats. Small Rumin Res. 1995;16:165-169.
12. Mauldin J: Is “two teats” the best answer? Jack & Anita Mauldin’s Boer goats (website): http://www.jackmauldin.com/management/two_teat_question.htm. Accessed December 10, 2010.
13. Panchadevi S.M., Pandit R.V. Milking males—two case studies. Indian Vet J. 1979;56:590-592.
14. Rieck G.W., et al. Gynakomastie bei einem Ziegenbock. II. Zytogeneticsche Befunde: XO/XY. Mosaik mit variablen Deletionen des Y-Chromosoms. Zuchthyg. 1975;10:159-168.
15. Wooldridge A., et al. Gynecomastic and mammary gland adenocarcinoma in a Nubian buck. Can Vet J. 1999;40:663-665.
16. Lofstedt R., Laarveld B., Ihle S. Adrenal neoplasia causing lactation in a castrated male goat. J Vet Intern Med. 1994;8:382-384.
17. Fubini S., Ducharme N., editors. Farm animal surgery. St Louis: Saunders, 2004.
18. Peer F.U., Bhattacharyya H.K. Studies on caprine gangrenous mastitis. Indian J Small Rumin. 2007;13:92-94.
19. Cable C.S., Peery K., Fubini S.L. Radical mastectomy in 20 ruminants. Vet Surg. 2004;33:263-266.
20. Youssef H.A. Mastectomy as a radical treatment for some prevalent udder affections in goats in Al-Gasseem. Assuit Vet Med J. 1999;41:181-193.
21. El-Maghraby H.M. Comparison of two surgical techniques for mastectomy of goats. Small Rumin Res. 2001;40:215-221.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree