Chapter 8. Diseases of the cornea and sclera
CHAPTER CONTENTS
Congenital and hereditary abnormalities203
Feline eosinophilic keratitis (proliferative keratitis)213
Equine eosinophilic keratitis, equine superficial corneal sequestrum, or equine indolent ulcer216
Feline corneal sequestrum (feline corneal necrosis, cornea nigrum)217
Corneal sequestration in other species218
Recurrent erosion syndrome (indolent ulcer, Boxer ulcer, spontaneous chronic corneal epithelial defects)219
Fungal keratitis, equine and other species222
Keratomalacia, collagenolytic keratitis, melting corneal ulcer223
Corneal perforation226
Corneal lysis, perforation, and iris prolapse with epithelialization in young cats226
Early life corneal perforation, early life trauma syndrome, and anterior chamber collapse syndrome227
Corneal epithelial inclusion cyst228
Inflammation disrupting the endothelium, endotheliitis228
NORMAL CORNEAL ANATOMY
The tear film
• The normal tear film is not visible using standard histopathology techniques
• Functions of the tear film include:
▪ Prevention of desiccation of the corneal epithelium and anterior stroma
▪ Distribution of oxygen and nutrients consumed by the corneal epithelium and anterior stroma
▪ Protection of the ocular surface:
– By trapping and flushing away particulate foreign material
– By immunoglobulins and other, non-specific anti-microbial proteins and peptides within the tear film, such as lysozyme and lactoferrin
– By providing a medium by which inflammatory cells can gain access to the tissues of the anterior cornea
▪ Provision of a glass-like smooth air-tissue interface that is essential for the optical clarity of the cornea
– Allows effective light refraction and the formation of a high quality focused image on the retina
• The components of the tear film
▪ The mucous layer
– Secreted predominantly by conjunctival goblet cells, with lesser contributions by the Harderian gland where present, corneal and conjunctival epithelial cells
– Attaches to the anterior surface of the corneal epithelium and adsorbs water, stabilizing the tear film
▪ The aqueous layer
– Secreted by the lacrimal gland, the nictitans gland and dispersed lacrimal glandular tissue in the conjunctival fornix
– The aqueous component of the tear film is the vehicle for delivery of oxygen and nutrients to the anterior cornea
▪ The lipid layer
– Secreted by the tarsal (meibomian) glands at the lid margin
– Forms an outer layer which slows evaporation and allows the even spread of the tear film over the ocular surface.
The corneal epithelium (Fig. 8.1)
Functions
• A physical barrier excluding pathogenic organisms
▪ The corneal epithelium is capable of rapid response to injury, covering an epithelial deficit by a process of relaxation of intercellular connections and vigorous cellular migration
• A lipophilic barrier to the absorption of hydrophilic substances
▪ This is the principle behind the use of the hydrophilic dye, fluorescein to detect defects in corneal epithelial integrity (corneal ulceration)
• The afferent branch of the corneal blink reflex
▪ Unsheathed nerve endings are distributed within the corneal epithelium
• The contribution of the cornea to the maintenance of immune privilege in the eye, and to the inhibition of neovascular proliferation within the corneal stroma.
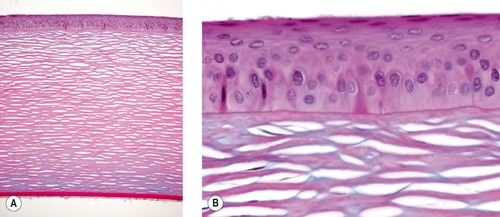 |
| Figure 8.1 Normal cornea. (A) Low magnification photomicrograph showing a normal canine cornea (Alcian blue PAS stain). (B) Photomicrograph of the normal corneal epithelium and superficial stroma. |
Components
Squamous cells
• Two or three superficial layers of non-keratinized cells
• Microvillae/microplicae present on these cells are postulated to help ‘anchor’ the tear film to the corneal surface
• The number of layers of this cell type is increased in larger species, e.g. ungulates and in species whose eyes maintain function underwater.
Wing cells
• Two or three layers of polyhedral cells
• The number of layers of this cell type is increased in larger species, e.g. ungulates and in species whose eyes maintain function underwater.
Basal cells
• A single layer of columnar cells, attached to the basal lamina by hemidesmosomes and to neighboring cells by desmosomes
• Hemidesmosomes attach to anchoring fibrils in the basal lamina
• The basal cell layer rests flat on the basal lamina with no undulation in contrast to most of the surface epidermis.
The corneal epithelial basal lamina and the superficial stroma
The basal lamina of the corneal epithelium and the superficial stroma are structures that play an important role in corneal wound healing and several disease processes that will discussed later in this chapter.
The lamellar corneal stroma
Functions of the lamellar stroma are:
• The maintenance of structural integrity, rigidity and the precise curvature of the globe
▪ The rigidity of the globe is essential to maintain precisely defined physical properties that are required to focus light on the retina. This rigidity relies on the tensile properties of the collagenous corneal stroma and the sclera, and on the maintenance of a physiologically high intraocular pressure
▪ The cornea represents a major refractive interface of the eye, and any changes in its curvature or thickness will impact the focusing of light on the retina
• Optical clarity
▪ The optical clarity of the corneal stroma is dependant on the maintenance of a strict, orderly structural relationship between cells, collagen, and inter-cellular, glycosaminoglycans within a relatively dehydrated extra-cellular environment
– Loss of optical clarity occurs if there is a failure to maintain relative dehydration of the corneal stroma, as occurs with loss of epithelial barrier function, vascular leakage, or particularly with loss of endothelial function (as discussed below)
▪ The corneal stroma is an immune privileged tissue and it is avascular
– Loss of optical clarity occurs with even slight inflammation or vascularization.
Descemet’s membrane (Fig. 8.2)
Descemet’s membrane is the basal lamina of the corneal endothelium. It forms a thick smooth membrane with elastic properties in terrestrial mammals but it is very thin in pinnipeds, birds, and reptiles.
 |
| Figure 8.2 Normal posterior cornea. (A,B) Photomicrographs showing the normal posterior canine cornea (A, H&E; B, PAS). |
Corneal endothelium
• The key function of the corneal endothelium is to maintain the stroma in a state of relative dehydration
▪ The endothelium is a physical barrier to the movement of water into the corneal stroma from the anterior chamber
▪ The endothelium functions to actively pump water out of the corneal stroma into the anterior chamber against a pressure gradient
• The endothelium is a monolayer of polygonal cells lining the posterior cornea
• In most adult animals, the endothelium has a very limited ability to proliferate in response to cell loss. However, metaplasia into spindle cells, movement along a surface, repair of the endothelium may occur. Damage to the endothelium might also be a stimulus for collagen matrix production of duplication of the Descemet’s membrane.
Comparative Comments
• Although there is variation in the proteins and lipids, the functions and dynamics of the tear film are similar in humans and animal species. Indeed, rabbits and cats represent major experimental models used to study tear dynamics and deficiencies, relevant to humans
• There are close similarities between the anatomy and histology of the human cornea with the description given for other species. Bowman’s layer is seen in the primate cornea and in certain avian corneas but is not seen as a distinct layer in most of the domestic mammalian species.
CONGENITAL AND HEREDITARY ABNORMALITIES
Congenital abnormalities in corneal size and shape
Cornea globosa in Rocky Mountain horses (Fig. 8.3)
Cornea globosa has been reported in association with congenital ocular anomalies in the Rocky Mountain horse. In some affected horses the radius of curvature of the cornea appears to be shortened, leading to excessive anterior curvature and protrusion of the cornea. These horses have abnormally deep anterior chambers. Other features of this inherited complex of multiple ocular abnormalities are discussed in detail in Chapter 3.
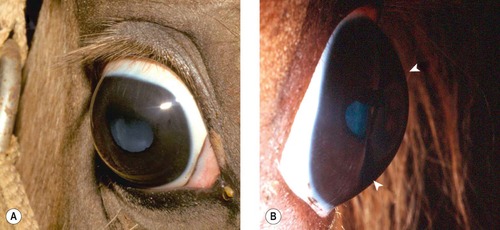 |
| Figure 8.3 Rocky Mountain horse. (A) The right globe has a megalocornea and a diffuse cortical cataract. (B) A lateral view illustrates the increased corneal curvature (arrows) in addition to the enlarged cornea, i.e. cornea globosa. |
Keratoconus, conical distortion of the anterior corneal curvature
Although animals will occasionally have corneal malformation with physical features of keratoconus, there are no documented animal models with equivalent morphology to this common human disease
Congenital corneal opacities
Peter’s anomaly (see also Ch. 3)
In this complex congenital abnormality, focal corneal opacity is caused by the attachment of uveal tissue within the posterior cornea, and associated defect in Descemet’s membrane (i.e. adherent leukoma). Attachments between the iris and cornea in dogs are also often referred to as persistent pupillary membrane but Peter’s anomaly is more accurate.
Dermoid (see also Ch. 7)
A congenital, focal lesion (choristoma), in which corneal tissue is replaced by fully differentiated tissue of another sort, typically skin.
Comparative Comments
Comparative Comments
• With regard to congenital and hereditary abnormalities, megalocornea is inherited as an X-linked recessive in humans, frequently as an isolated bilateral abnormality
• Congenital corneal opacities may result from anterior cleavage anomalies and may be associated with abnormalities of the iridocorneal angle and iris
• Other significant congenital anomalies that occur in man are congenital hereditary endothelial dystrophy, causing bilateral congenital corneal edema; posterior keratoconus, a central indentation of the posterior cornea; congenital corneal staphyloma, characterized by ectasia of part or all of the cornea; and sclerocornea, an abnormality resulting in bilateral opacification and vascularization of the cornea, leading to its resembling the adjacent sclera
• Keratoconus in humans is characterized in its early stages by focal disruptions of the epithelial basement membrane and Bowman’s layer, with subsequent bilateral central ectasia of the cornea
• Dermoids are congenital fibrovascular masses present within the cornea. They are believed to be caused by a failure of surface ectoderm to differentiate into normal corneal epithelium.
CORNEAL DYSTROPHIES AND DEGENERATIONS
Corneal epithelial dystrophies (Fig. 8.4)
Examples of corneal epithelial dystrophies in animals
• Corneal dystrophies are typically bilateral in presentation and a familial or breed predisposition is often documented or suspected
• Breed-related epithelial dystrophies are reported in dogs, these include:
▪ Superficial punctate keratopathy in the Shetland Sheepdog and Long Haired Dachshund
– Onset is typically in early adulthood, with varying progression throughout life. Affected animals typically present with multi-focal epithelial defects that lend an ‘orange peel’ appearance to the corneal surface, and are associated with small, round to annular opacities
– The cause of these lesions is poorly understood – although some consider this to represent a primary epithelial dystrophy, qualitative tear film disease and immune-mediated keratitis have also been proposed as underlying abnormalities in affected dogs
• Morphologic features reported in eyes affected by epithelial dystrophy include:
▪ Focal dysplasia of the basal lamina of the corneal epithelium
▪ Dyskeratosis and necrosis of corneal epithelial cells
• In the COPLOW collection, however, there are only two examples of corneal epithelial dystrophies and none in dogs
▪ One in a Basilisk lizard and one in a horse.
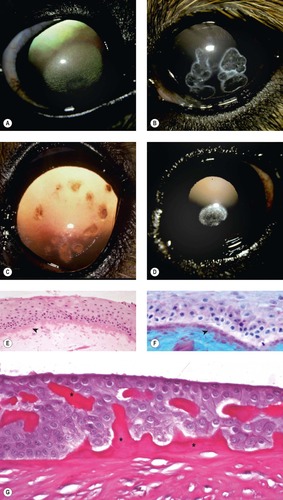 |
| Figure 8.4 Epithelial dystrophy and stromal lipid dystrophy. (A) Afghan Hound, 3 years old: bilateral elliptical, superficial stromal dystrophy was present. (B) Shetland Sheepdog corneal dystrophy, 3.5 years old: multiple superficial facets with associated lipid deposits were present in both corneas. (C) Shetland Sheepdog, 2 years old: bilateral focal dystrophies can be seen by retroillumination. (D) Cavalier King Charles Spaniel, 4 years old: axial opacities were present bilaterally. (E,F) Photomicrographs of suspected corneal epithelial dystrophy in a horse showing marked irregularity in the corneal epithelial basal lamina (arrows) (E, H&E; F, Alcian blue PAS). (G) Photomicrograph showing the corneal epithelium and dysplastic basal lamina (*) from a Basilisk lizard (PAS stain). |
Corneal stromal dystrophies and degeneration
Because penetrating keratoplasty is performed so often in humans, there is a much greater understanding of the pathologic basis of human corneal dystrophies. In contrast, corneal tissues from uncomplicated cases of suspected corneal dystrophy in domestic animals are seldom submitted to COPLOW.
Lipid dystrophy, crystalline stromal dystrophy, corneal lipidosis (Fig. 8.5)
Several commonly occurring, breed-related corneal lipid dystrophy syndromes are reported in dogs. Although in some breeds, the inherited basis of corneal disease has been established and histopathological findings published, affected corneal tissues are seldom submitted to the pathology laboratory as they are neither painful nor vision-threatening in most cases.
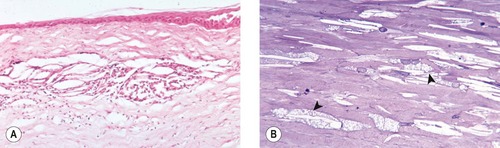 |
| Figure 8.5 Cholesterol deposits, canine. (A) Photomicrograph showing a cholesterol granuloma in the midsuperficial stroma. (B) Photomicrograph showing keratocytes with vacuolated cytoplasm due to increased lipid content (arrows) (toluidine blue stain on 1 µm plastic section). |
In pathology submissions, lipid deposition in the cornea is usually found as a condition secondary to inflammation, or mass lesions involving the limbus and/or cornea.
Characteristics of lipid dystrophy which distinguish it from acquired disease include:
• Bilateral, generally relatively symmetrical, nature of disease
• Breed predisposition that includes the Siberian Husky, Beagle, Cavalier King Charles Spaniel, Collie, German Shepherd, Shetland Sheepdog, Afghan Hound and Airedale
• Found in an otherwise clear cornea:
▪ Typically, there is no, or very limited, vascular in-growth and limited inflammatory response
• No defined relationship to underlying systemic metabolic disease
▪ However, abnormalities in serum lipids and lipoproteins may affect the onset and progression of corneal opacities in dogs with primary corneal lipid dystrophies
▪ Likely represents a primary abnormality in the metabolism of keratocytes.
Morphology of corneal lipid dystrophy
• The obvious lesion is free cholesterol between stromal lamellae, most often involving the anterior stroma. This is recognizable in paraffin-embedded sections as oblong spaces, including characteristic, needle-shaped, acicular clefts. Often lipid is evident within or surrounded by macrophage cells (cholesterol granuloma)
• Careful observation of surrounding keratocytes reveals that there are lipid vacuoles in the cytoplasm of keratocytes
• Lipid deposits whether intracellular or within the stroma will not be directly observable by standard paraffin histologic techniques because the lipid is dissolved in xylene and in paraffin during processing.
Acquired corneal lipid deposits (Fig. 8.6)
• Lipid keratopathy
▪ Associated with vascularization of the cornea (that may be recognized prior to, or following, lipid deposition)
▪ Lipid deposition may occur in the corneal stroma at the margins of localized proliferative diseases such as melanocytoma, nodular granulomatous episcleritis or focal trauma, or may be more diffuse when associated with keratitis or other generalized anterior segment inflammation (scleritis, uveitis)
▪ There may, or may not, be an association with abnormal systemic lipid metabolism, such as endocrine disorders or excessive amounts of dietary lipids
• Arcus lipoides corneae
▪ Originates near the limbus
▪ Bilateral
▪ Initially corneal blood vessels are absent, but vascularization is a subsequent response to the presence of lipid and typically is accompanied by the presence of lipid-filled macrophages. Indeed, the extent of vascularization and inflammation can modify further lipid deposition or removal
▪ No causal association with local proliferative and inflammatory corneal disease
▪ Associated with systemic metabolic diseases, such as hypothyroidism
▪ In domestic and laboratory rabbits, feeding of diets high in cholesterol, or genetic hyperlipidemia (‘Watanabe’ rabbits), has been associated with lipid infiltration of the cornea (lipid keratopathy, arcus) as well as in other ocular tissues
– A distinct form of anterior corneal (epithelial and epithelial basement membrane) dystrophy has also been reported in Dutch Belted rabbits
▪ An association between serum lipids and corneal lipid deposition has also been widely recognized in captive tree frogs.
 |
| Figure 8.6 Lipid keratopathy and corneal fatty degeneration, clinical. (A) Golden Retriever cross, 3 years old: episcleritis and hypothyroidism resulted in this bilateral condition. (B) Golden Retriever, 4 years old: blood vessels are present at the limbus around this dense lipid deposit. (C) Labrador Retriever, 4 years old: the corneal lipid is at the advancing margin of the scleral shelf melanoma. (D) Rottweiler, 4 years old: diffuse scleritis, limbal edema and corneal vascularization are associated with the lipidosis (arrow). |
Mineral stromal dystrophy and degeneration (Fig. 8.7)
As with lipid, mineralization of the corneal stroma is most often encountered as a secondary process within our pathology collection. However, there are a few submissions with mineralization of the stroma in which there is either no underlying inflammation evident, or the degree of mineralization appears excessive relative to the limited inflammation present.
 |
| Figure 8.7 Corneal mineralization. (A) Miniature Dachshund, 11 years old: this is an early case with no vessels associated with the calcium deposit visible by retroillumination. (B) Cockapoo, 15 years old: the arrow points to a deep stromal crater within the calcium deposits. (C) Yorkshire Terrier, 16 years old: there is a large area of mineralization with corneal vessels superior. (D) Terrier mix, 13 years old: a central descemetocele is present with corneal vessels 360°. (E,F) Photomicrographs of canine corneal epithelium showing mineralization of the basal lamina and superficial stroma (band keratopathy) (E, H&E; F, elastin stain). (G) Photomicrograph of an equine corneal epithelium showing mineralization of the basal lamina. (H) This equine corneal epithelium shows marked mineral in the basal lamina and superficial stroma (von Kossa stain). |
Corneal stromal mineralization in horses
• This presents as distinct areas of superficial corneal opacity
• The mineralization is confined to the superficial stroma, with minimal or no inflammation present
• Cases from the COPLOW collection do not have a history of metabolic disease and there is no obvious relationship to diet or to any particular breed or age.
Band keratopathy (Fig. 8.8)
• Band keratopathy represents a nonspecific degenerative process, and is named for its appearance as an approximately axial, horizontal band that lies within the inter-palpebral fissure
• Band keratopathy may also be seen in animals that have underlying disease leading to hypercalcemia (metastatic calcification)
• Corneal calcification has also been reported following topical therapy with steroid-phosphate preparations
• Morphologic characteristics
▪ Superficial corneal stromal mineralization or basal laminar mineralization
▪ The mineralized segment often becomes separated from the epithelium because the cornea continues to grow and surround the mineralized fragment, which remains as a linear, mineralized tissue fragment embedded in the anterior stroma.
▪ Because the mineralized tissue was originally basal lamina, the remnant fragments stain positively with PAS.
 |
| Figure 8.8 Band keratopathy. (A) Photomicrograph of a canine anterior cornea showing thickening and basophilia of the epithelial basal lamina typical of the mineralization seen in band keratopathy (arrow). (B) Photomicrograph of a canine anterior cornea showing mineralized basal lamina in an area of epithelial loss (arrow). (C) The mineralized basal lamina and anterior stroma in a dog cornea stain black with von Kossa stain. |
Calcareous corneal degeneration, in aged dogs
• Slowly progressive disease
• Ultimately bilateral although not necessarily symmetrical in onset
• The diseased stroma displays white deposits that are gritty in texture
• Can progress to deep stromal ulcers or even corneal perforation
• Segmental mineralization of epithelial basal lamina (band keratopathy) and mineralization of the deeper stroma
• Affected animals may have no history of underlying ocular disease or evidence of systemic hypercalcemia.
Comparative Comments
Dystrophies of the cornea are defined as primary, inherited bilateral disorders and are classified according to the layer of the cornea most involved. These have been described, classified, and studied more fully in man than in other species.
• Although Cogan’s microcystic or map-dot-fingerprint dystrophy is probably the most common, specimens are rarely received in the eye pathology laboratory
– The chief histopathologic findings are a thickened basement membrane that extends into the epithelium, with associated abnormal epithelial cells and microcysts
• Lipid dystrophy was discussed among the corneal stromal dystrophies in nonhuman species
– In man, the most common form of lipid deposit seen is arcus senilis, which is a primary condition
– In Schnyder’s crystalline dystrophy, a rare primary lipoidal degeneration, cholesterol crystals are noted within keratocytes and adjacent stroma
– Also cholesterol and neutral fats are sometimes found as secondary lipid deposits following corneal inflammatory disease with new blood vessel formation
• The major human stromal dystrophies are:
– Granular dystrophy, in which keratohyaline deposits are noted
– Lattice dystrophy, in which fine-branching amyloid opacities are seen
– Avellino dystrophy (combined granular/lattice dystrophy)
– Macular dystrophy, in which there is proteoglycan deposition in all layers of the cornea except the epithelium
• Band keratopathy in humans conforms in appearance to the descriptions given here for other species
– Band keratopathy may follow any chronic local corneal disease or occur in association with systemic hypercalcemic states and in eyes with longstanding chronic inflammation.
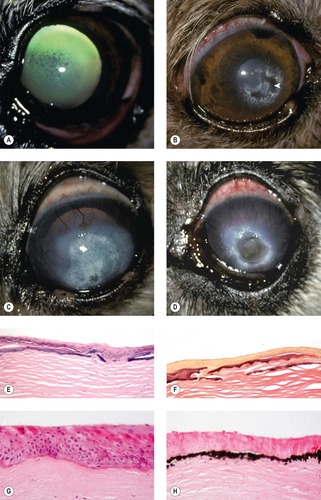
Corneal endothelial dystrophies and degeneration (Fig. 8.9)
As with the other corneal dystrophies, it can be difficult to distinguish true endothelial dystrophy from acquired endothelial disease, which is much more commonly encountered. There are, however, several canine examples of endothelial dystrophy in the COPLOW collection.
Comparative Comments
• Endothelial dystrophy with multifocal defects in Descemet’s membrane in dogs less than 6 months old
▪ There are three such cases with bilateral disease in the COPLOW collection in three different breeds
▪ Euthanasia was elected in all three cases because of the severe, blinding nature of the corneal disease
▪ The affected corneas were markedly thickened and opaque
▪ Histopathology shows a relatively normal endothelium, but with multiple defects in Descemet’s membrane. Within these defective areas, endothelial cells may be seen to ‘colonize’ the posterior stroma
• Breed related corneal endothelial dystrophy and spontaneous corneal endothelial degeneration (attenuation)
▪ Boston Terriers and Chihuahuas are known to have a breed-related corneal endothelial dystrophy
▪ Mature adult dogs are typically presented with bilateral corneal edema that begins temporally and progresses to involve the entire cornea. Secondary corneal ulceration, due to rupture of epithelial bullae, is a common complication of profound corneal edema
▪ Affected dogs have corneal stromal edema and endothelial cell attenuation as a primary disease, without an identifiable underlying pathogenic mechanism
▪ In the COPLOW collection there are 12 cases which are considered to represent breed-related corneal endothelial dystrophy
– Five Boston Terriers, three Chihuahuas, three Puli, and one Poodle
▪ In all cases, the only morphologic finding was corneal endothelial cell attenuation, with profound corneal stromal edema and no apparent reason for the endothelial cell degeneration.
Comparative Comments
The endothelial dystrophies in humans appear different from those described above in young dogs. In man, the endothelial dystrophies have three factors in common:
1. The endothelial cells become sparse and irregular.
2. The abnormal endothelial cells produce excess collagen posterior to Descemet’s membrane, causing a multilaminar structure.
3. Excrescences form on Descemet’s membrane.
The most common endothelial dystrophy in humans is Fuchs’ dystrophy, which constitutes the common indication for corneal surgery for a corneal dystrophy.
 |
| Figure 8.9 Corneal endothelial dystrophy. (A) Chihuahua, 10 years old: bilateral temporal edema was present. (B) Boston Terrier, 8 years old: the arrow points to epithelial bullae superficial to the stromal edema. (C) Boston Terrier, 7 years old: the arrows delineate the keratoconus resulting from severe corneal edema. (D) Dachshund, 11 years old: the arrow points to the edge of a deep corneal crater (facet), secondary to the edema and previous ulcer. (E) Gross photograph of a canine globe with endothelial dystrophy showing marked corneal stromal thickening. (F) Clinical photograph of a canine eye with dense corneal edema resulting in a blue to white opacity secondary to endothelial disease. (G,H) Photomicrographs showing the corneal endothelium of the eye in (E). The endothelium is attenuated and there are eosinophilic intracytoplasmic inclusions (arrows). |
Secondary degenerative endothelial diseases (Fig. 8.10)
Pathogenic mechanisms
• Postoperative/iatrogenic (see Ch. 4)
▪ Corneal endothelial attenuation is not uncommon as a postoperative complication following intraocular surgery, most notably, cataract surgery
• Any other disease process associated with tissue contact with the corneal endothelium such as anterior synechiae, or uveal masses such as neoplasms or cysts
▪ Inflammatory disease may also play a role in endothelial dysfunction in some of these disease processes
• Advanced age
▪ Corneal endothelial density reduces progressively with age and may ultimately fall below a critical density that is needed to maintain relative dehydration of the corneal stroma
• Glaucoma
• Lens luxation
▪ Focal edema due to direct contact between the displaced lens and corneal endothelium
▪ Diffuse edema may be associated with secondary glaucoma.
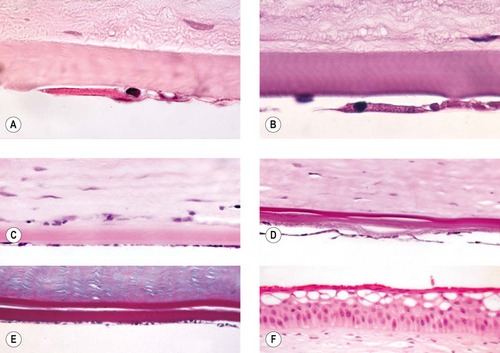 |
| Figure 8.10 Corneal endothelial disease. (A,B) Photomicrographs of attenuated and abnormal corneal endothelium (B, PAS stain). (C) Photomicrograph showing attenuated corneal endothelium in a dog. (D) Retrocorneal membrane and doubling of Descemet’s membrane in a dog cornea (PAS stain). (E) Prominent doubling of Descemet’s membrane. (F) Corneal epithelium showing microvesicular change suggestive of corneal edema. |
Morphologic features of corneal endothelial degeneration
• The early changes are confined to endothelial attenuation, characterized by a reduction in cell density that is recognized as a ‘spreading’ of each individual endothelial cell, such that the distance between sampled nuclei is larger and the cell thickness is reduced
• Retrocorneal membrane formation
▪ More advanced or longer standing cases demonstrate spindle cell metaplasia of the endothelium, with or without collagen formation
• Duplication of Descemet’s membrane
▪ This phenomenon is seen in dogs and cats, but not in horses, after intraocular surgery, blunt trauma, lens luxation and in other conditions where the endothelium might be ‘stressed’
▪ Descemet’s is usually duplicated; with the new, posterior, Descemet’s being approximately the same thickness as the original, anterior Descemet’s membrane
▪ The mechanism responsible for duplication of Descemet’s membrane is unknown.
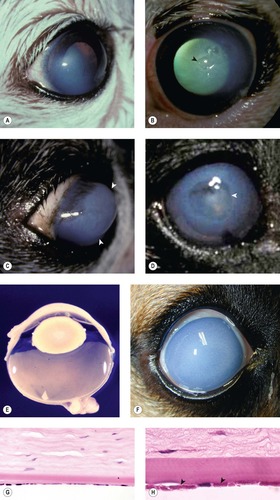
Severe corneal edema (corneal hydrops) associated with Descemet’s membrane rupture (Figs 8.11, 8.12)
• Most of the examples of this condition in the COPLOW collection are in cats (five cats), although the condition has been reported in other species including horses
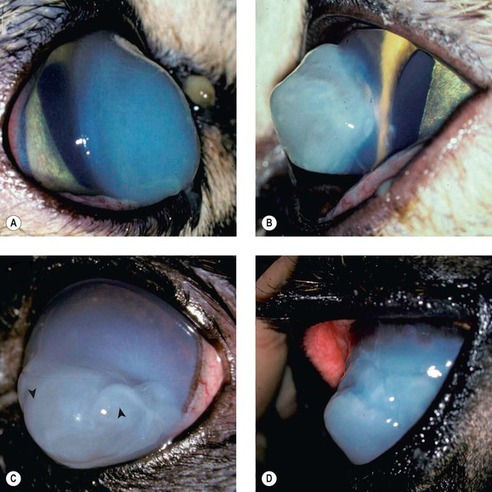 |
| Figure 8.11 Corneal hydrops, clinical. (A) DSH, 1 year old: the lateral view shows the increased corneal curvature due to the severe edema. (B) DSH, 2.5 years old: the protruding cornea is irregular in contour. (C) Pomeranian, 5 years old: diffuse stromal edema is present with associated large epithelial bullae (arrows). (D) Thoroughbred, 3 years old: the acute edema caused the irregular conical cornea. |
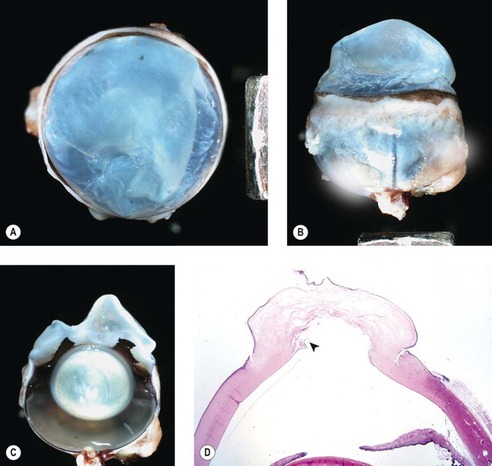 |
| Figure 8.12 Feline corneal hydrops, pathology. (A–C) Three gross photographs of the same cat eye showing corneal hydrops and misshapen, markedly edematous corneal stroma. (D) Low magnification photomicrograph of the same cat eye showing edema, epithelial separation and a defect in Descemet’s membrane (arrow). |
• The rapidity of onset is reflected in the other term for the condition, ‘acute bullous keratopathy’
• The condition is characterized by rapidly developing, dense, relatively well-circumscribed corneal edema, with large coalescing bullae within the stroma and gross distortion of the corneal profile. Pronounced conical or globular forward protrusion of the anterior profile of the cornea ensues
• Histologically, rupture of Descemet’s membrane is a consistent finding, and is associated with marked stromal edema with little or no inflammation.
CHRONIC KERATITIS, SUPERFICIAL
Chronic keratitis represents a non-specific end result of many diverse pathogenic pathways, including:
• Physical irritation from contact with hair, inflammatory or neoplastic masses, or airborne particulate material
• Desiccation caused by:
▪ Exophthalmos or buphthalmos which impede eyelid closure and leads to inadequate distribution of the tear film
▪ Inability to blink related to neurologic lesions affecting the facial nerve
▪ Loss of corneal sensitivity related to lesions affecting the ophthalmic branch of the trigeminal nerve, with failure to initiate a protective blink or lacrimal response to irritation
▪ Disorders resulting in decreased secretion of the aqueous component of tears
– Chronic inflammation of the lacrimal and/or nictitans gland
– Atrophy of these glands or obstruction of their ducts, secondary to trauma, inflammation, chemical injury, or radiation damage
– Conjunctival xerosis or fibrosis
– Neurogenic disease
▪ Recurrent episodes of corneal ulceration for whatever reason
▪ Keratitis due to specific corneal pathogens, e.g., Moraxella bovis, Feline herpesvirus-1
▪ Immune-mediated disease
– The most commonly encountered breed-related condition being chronic superficial keratitis, previously known as pannus or Überreiter’s syndrome, in dogs (see below).
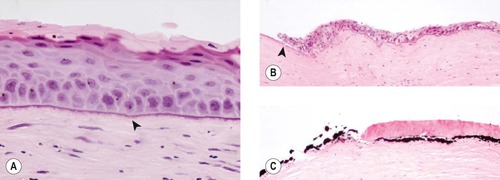
Morphologic features of chronic keratitis (Fig. 8.13)
The epithelial response (epidermalization) is dependant on an intact limbal epithelium because the stem cells for the cornea reside only in the limbus. The components of the epithelial response in chronic superficial keratitis are:
• Hyperkeratosis
• Acanthosis
• Rete ridge formation
• Melanosis
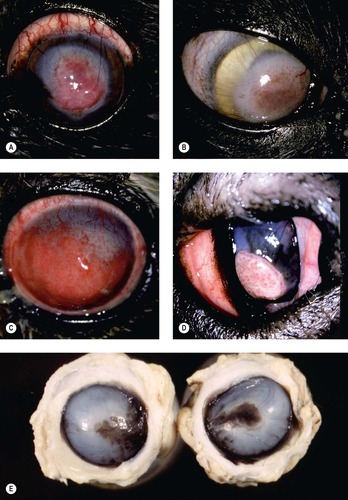 |
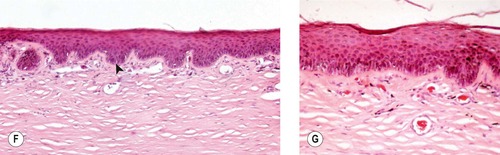 |
| Figure 8.13 Superficial chronic keratitis. (A) Shih Tzu, 6 years old: this was the result of previous keratomalacia. (B) DSH, 7 years old: FHV-1 with secondary bacterial infection resolved but lead to the development of a dense fibrovascular scar. (C) Shih Tzu, 11 years old: poorly controlled dry eye syndrome led to severe chronic superficial keratitis. (D) Chinese Shar-Pei, 1 year old: this resulted from primary entropion. (E) Gross photograph of both globes from a Pug dog showing melanin pigment deposition in superficial chronic pigmentary keratitis. (F,G) Photomicrographs showing epithelial thickening, rete ridge formation (arrow), superficial stromal fibrosis and vascularization, and minimal lymphocytic infiltration in canine superficial chronic keratitis. |
The stromal response includes:
• Vascular in-growth from the limbus
• Reorganization of stromal collagen, with scarring
• Melanosis
• Inflammatory cell infiltration
▪ Which depends on the stage of disease, prior therapy and the nature of the inflammation locally at the time of sampling
The basal lamina response:
• Marked thickening of the basal lamina may occur in response to repeated ulceration and re-epithelialization.
CHRONIC SUPERFICIAL KERATITIS (CSK, PREVIOUSLY TERMED PANNUS OR ÜBERREITER’S SYNDROME) (Fig. 8.14)
• This is a chronic, progressive, immune-mediated inflammatory condition which spreads from the limbus across the superficial cornea, typically commencing in the temporal quadrant
• This bilateral condition has a predilection for the adult German Shepherd dog, Tervuren, Greyhound, Border Collie and Siberian Husky, although other breeds may be affected sporadically
• Ultraviolet light exposure has been implicated as a factor in the pathogenesis, and related environmental factors including altitude, sunlight exposure and dust may all adversely impact disease onset and severity.
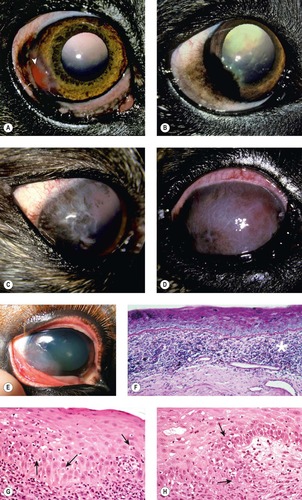 |
| Figure 8.14 Canine chronic superficial keratitis (degenerative pannus). (A) German Shepherd Dog, 4 years old: the arrowhead points to the early temporal limbal vascularization. (B) Belgian Tervuren, 2 years old: temporal corneal pigment is the more remarkable finding in this right eye. (C) Greyhound, 4 years old: thin corneal vessels, lipid and pigment are present. (D) German Shepherd Dog, 6.5 years old: the entire cornea is vascularized in this advanced case. (E) Clinical photograph showing the superficial corneal proliferative reaction advancing over the temporal limbus. (F) Photomicrograph showing the typical interface inflammatory infiltrate (*). (G,H) Photomicrographs showing necrosis of individual epithelial cells typical of the disease (arrows). |
Morphologic features of chronic superficial keratitis
• Lichenoid (interface) lymphoplasmacytic inflammation
• Superficial stromal fibrovascular proliferation and scarring, with subsequent melanosis
• Increased epithelial cell apoptosis
• Increased epithelial cell mitotic activity, but no associated epithelial erosion or ulceration.
The prognosis for achieving disease control and preserving vision may be poorer in those animals that show rapid onset of disease in early adulthood. However, most affected dogs show a favorable response to topical immunosuppressive therapy.
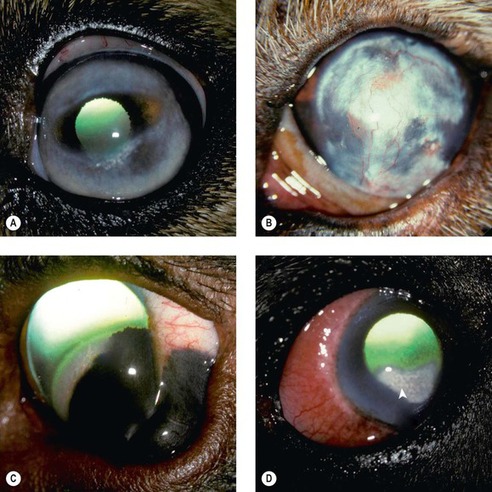
FELINE EOSINOPHILIC KERATITIS (PROLIFERATIVE KERATITIS) (Figs 8.15, 8.16)
Feline eosinophilic keratitis is a relatively commonly encountered disease in companion animal veterinary practice. However, the disease is under-represented in a pathology collection because it is usually diagnosed on the basis of clinical and/or cytological findings alone. There are only 24 cases in the COPLOW collection, most of which are keratectomy specimens.
• Feline eosinophilic keratitis typically extends across the limbus as a kerato-conjunctivitis. Eosinophilic conjunctivitis and eyelid margin erosive lesions may also be seen in the absence of significant corneal disease
• Lesions are most often unilateral, although bilateral involvement does occur
• Grossly the disease appears as a vascularized, peri-limbal, raised, proliferative lesion, with an irregular surface that has characteristic superficial plaques of white material. Although localized, corneal involvement can be extensive
• An association between eosinophilic keratitis and Feline herpesvirus-1 infection has been demonstrated in some affected cats but virologic studies have not defined a consistent relationship between the presence of this virus and eosinophilic keratitis in all cases
▪ Eosinophilic/proliferative keratitis, and FHV-1 stromal keratitis likely represent similar immune-mediated disorders of the feline cornea, that may or may not share the same inciting factors
• Eosinophilic keratitis generally shows a dramatic response to topical immunosuppressive therapy
• Morphologic features of feline eosinophilic keratitis:
▪ The epithelium is usually intact but, occasionally, an ulcerated surface may be lined by granular, acellular protein that likely represents granules liberated from eosinophils and/or mast cells. In addition to focal absence or thinning of the epithelium, hypertrophy and hyperplasia of the epithelium may also be seen
▪ The normal superficial lamellar stroma may be completely effaced
▪ Associated new corneal blood vessels can be large and may cross the lesion obliquely
▪ The inflammatory infiltrate is lympho-plasmacytic with variable numbers of eosinophils and mast cells
– Eosinophils often do not dominate the infiltrate and they may even be present in very small numbers.
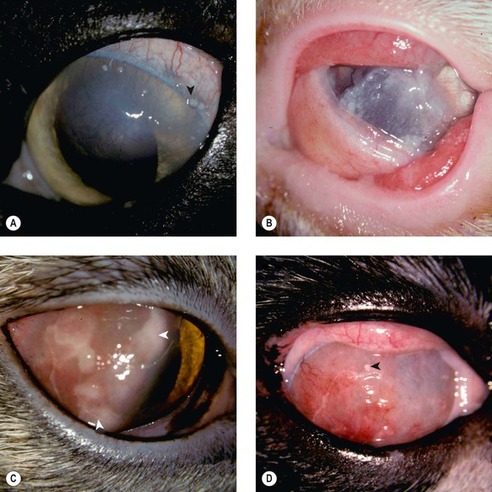 |
| Figure 8.15 Feline eosinophilic keratitis, clinical. (A) DSH, 14 years old: the arrow points to one of the many superficial white plaques typical of the disease. (B) DSH, 4 years old: a thick, white, gritty plaque covers most of the cornea. (C) DSH, 1.5 years old: homogeneous white material (arrows) is present over the highly vascularized cornea. (D) DLH, 8 years old: the arrow points to a superficial white plaque on the vascularized cornea. |
 |
| Figure 8.16 Feline eosinophilic keratitis, pathology. (A) Low magnification photomicrograph of a feline cornea showing the features of eosinophilic keratitis. There is abrupt effacement of anterior lamellar stroma by heavily vascularized, loose edematous connective tissue and an inflammatory cell infiltrate (arrows). (B) Higher magnification photomicrograph showing the surface epithelium with deep epithelial pegs extending into the loose stroma. (C) In this photomicrograph, an ulcerated surface is carpeted by hypereosinophilic protein material (*), a rare feature of feline eosinophilic keratitis. |
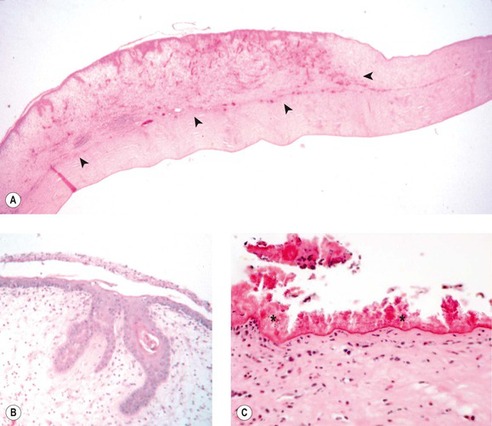
EQUINE EOSINOPHILIC KERATITIS, EQUINE SUPERFICIAL CORNEAL SEQUESTRUM, OR EQUINE INDOLENT ULCER (Fig. 8.17)
These three conditions share similar clinical and morphologic features to an extent that, in the cases submitted to COPLOW, it has not been possible to distinguish one syndrome from another. There are 16 cases in the COPLOW collection, with clinical or morphologic features suggesting one or the other of these syndromes. However, samples are seldom submitted from cases with a clinical diagnosis of eosinophilic keratitis for histopathologic evaluation, because the diagnosis is frequently made by cytology.
• These conditions present as superficial recurrent or non-healing ulcers affecting the peripheral cornea
• Similar clinical and histopathological findings have been reported in horses with ocular onchocerciasis, in which microfilaria have been identified within the corneal or conjunctival tissues
• Morphologic features of the disease include:
▪ A failure of epithelial attachment
▪ A very thin hyper-eosinophilic band of material between the unattached epithelium and the more normal stroma
▪ Eosinophils and other inflammatory cells are seldom seen in samples submitted for pathology
– This may be because the cases with abundant eosinophils are readily diagnosed based on clinical appearance and cytologic findings.
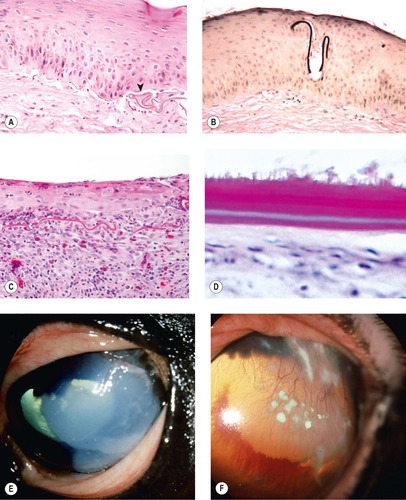 |
| Figure 8.17 Equine superficial sequestrum/eosinophilic keratitis. (A) Photomicrograph of the epithelium from a horse with equine eosinophilic keratitis or superficial sequestrum. The thin membranous sequestered protein material is wrinkled at the base of the epithelium (arrow). (B) A fragment of sequestered membrane (black) is entrapped by the epithelium that has reformed around it (Verhoeff’s elastic stain). (C) Photomicrograph showing a poorly attached epithelium, a wrinkled remnant of sequestered membrane and an eosinophilic infiltrate, an infrequent finding in a pathology specimen. (D) Photomicrograph showing a thick and doubled sequestrum membrane on the surface of an ulcerative lesion. (E) Thoroughbred, adult: the large white gritty superficial deposit was also fluorescein-positive. (F) American Quarter Horse, 11 years old: superficial corneal vessels and multiple white precipitates are present. Conjunctival cytology demonstrates many eosinophils.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|