Diseases of the Cardiovascular and Hemolymphatic Systems
The Cardiovascular System
Clinical Evaluation
Physical Examination
Physical examination of the cardiovascular system involves examination of mucous membranes for color, refill time, and moistness; thoracic palpation and auscultation, assessment of arterial pulse quality via the median or medial saphenous arteries; assessment of jugular filling; and palpation of the ventrum for edema. Common ancillary tests include blood analysis, blood pressure measurement, thoracic radiography, electrocardiography, and echocardiography. The areas suitable for cardiac auscultation lie conveniently within the short-fleeced axilla, under the forelimb above the elbow. On the left side, the pulmonic valve is heard best over the third intercostal space, under the triceps brachii, whereas the aortic valve area is located more dorsally at the fourth intercostal space. The mitral valve area is located more ventrally on the left hemithorax at the costochondral junctions over the fourth or fifth intercostal space, and the tricuspid valve is heard best on the right hemithorax over the fourth intercostal space at the caudal edge of the triceps brachii. Areas over all valves should be palpated for the presence of a thrill. Healthy adult camelids in the Oregon State University herd have resting heart rates between 48 and 72 beats per minute (beats/min), with rare individuals spiking up to 84 beats/min. This range is slightly lower than the 60 to 90 beats/min or higher rates that are sometimes cited. Adults with higher heart rates are likely to be excessively stressed, volume depleted, or otherwise compromised. Neonates typically have heart rates varying from 90 to 120 beats/min, although rates up to 140 beats/min may be found in some newborns. Juveniles typically will have heart rates less than 100 beats/min by age 1 month, and this declines to the adult range by age 1 year.1
The first and second heart sounds should be heard clearly on the left side of the thorax in all but the most obese camelids. These sounds are more difficult to discern on the right side but should be audible in an adequately restrained camelid in a quiet environment. With careful auscultation, soft third and fourth sounds are discernible in some healthy camelids. Loud S3 or S4 heart sounds are usually indicative of serious cardiac disease, reflecting the presence of ventricular enlargement and ventricular dysfunction. Physiologic and innocent murmurs are often identified in adult and immature camelids. Anemic, systemically ill, and even apparently healthy camelids may exhibit soft, nonpathologic systolic murmurs (≤grade 3 of 6) over the left heart base. These murmurs are usually crescendo–decrescendo, ejection-type murmurs occupying midsystole, with preservation of the first and second heart sounds. Scansen and co-workers recently identified high-velocity and turbulent flow in the branch pulmonary arteries of several young crias and offered an elegant explanation for some of the innocent murmurs identified in immature camelids.2 The murmur and the flow disturbances resolved within a few months, which suggests that this finding is benign, therein resembling the condition of peripheral pulmonary stenosis seen in human infants. Often, however, the precise cause of these soft murmurs is not apparent even with the aid of color flow echocardiographic evaluation.
The lymphatic system is often conveniently assessed by examining the size of peripheral lymph nodes, particularly when they are enlarged because of inflammation or neoplasia. Moreover, it is not uncommon to identify disorders of the lymphatic system by inference when lymph accumulates in a body cavity or regional pitting is noted in an extremity. The easiest lymph nodes to find in normal camelids include the prescapular nodes located near the base of the neck and the superficial inguinal nodes located in the inguinal region close to the ventral body wall. Other peripheral nodes are difficult to locate in healthy camelids because they often comprise a collection of small nodules. Emaciation or pathologic node enlargement facilitates successful palpation of the submandibular, retropharyngeal, and popliteal nodes, which are often very hard to identify in healthy animals.
Electrocardiography
Electrocardiograms (ECGs) are primarily recorded to elucidate the nature of an auscultated rhythm disturbance. A standardized six-lead ECG may be easily obtained in the standing camelid by attaching two electrodes above the olecranon on the forelimbs and the remaining two electrodes just above the patellas on the hindlimbs. Leads are attached with alligator clips and wetted with alcohol. Clipping the fleece is usually not necessary. It is often preferable to obtain ECGs in young crias by restraining them in right lateral recumbency. In either circumstance, ECG tracings are most easily interpreted when a paper speed of 50 millimeters per second (mm/s) is selected for the recording. It is useful to complement the limb leads with a rhythm strip that is recorded by using a base–apex lead; the rhythm strip is obtained by repositioning the right front leg electrode to the base of the neck on the right and by moving the left front leg electrode to a location over the cardiac apex on the left side of the chest. This lead often provides the largest amplitude complexes thereby facilitating cardiac rhythm analysis.3
Normal ECG reference values for camelids have been reported in several published studies (Figure 36-1).4–6 In the recent report by Kraus and coworkers, heart rate was seen to vary from 60 to 100 beats/min with a mean heart rate of 80 beats/min.6 The mean (+/− standard deviation [SD]) duration of the electrical events were reported as follows: P waves: −40 milliseconds (ms; +/− 12); PQ interval: 150 ms (+/− 33); QRS complex: 50 ms (+/− 8); Q–T interval: 360 ms (+/− 60); ST segment: 230 ms (+/− 46). QRS morphology was found to be extremely variable. The mean polarity of the QRS complexes was negative in 60% of the lead I recordings, in 56% of the lead II recordings, and in 51% of the lead III recordings. The QRS complex was least variable for lead V10, where the QRS complexes were positive in 88% of the animals examined. The amplitudes of the R and S waves tended to be low in the limb leads, as often noted in herbivores. Values obtained from mean electrical axis (MEA) estimations were extremely variable but were directed mainly to the right and in a cranial direction. In contrast to humans and small animals, ECG recordings obtained from camelids provide little information about the overall size of the heart or the enlargement of specific chambers. The Purkinje fiber network of camelids penetrates completely through the wall of the ventricles from the endocardium to the epicardium, as in sheep, resulting in a pattern of activation that effectively obscures the hallmarks of chamber enlargement seen in humans and smaller mammals such as the dog and the cat.4,7 In our experience, neither the recorded voltage nor the estimated MEA appears to be useful for the detection of chamber enlargement.
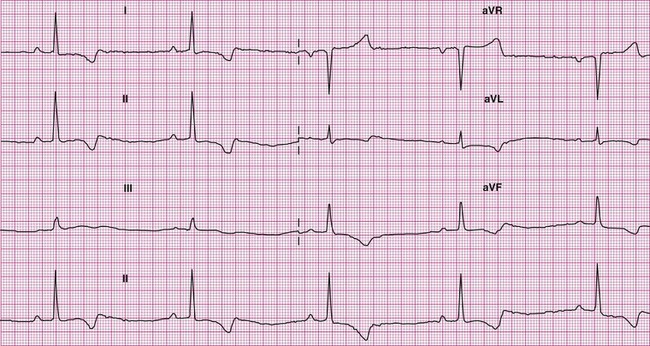
Figure 36-1 As in other species with extensive arborization of Purkinje fiber throughout the myocardium, marked variability of R wave amplitudes and the mean electrical axis is seen. This 6-lead electrocardiogram was recorded from a normal adult male alpaca.
Cardiac rhythms observed in healthy camelids include sinus rhythm and, more commonly, sinus arrhythmia with an occasional animal showing first or low grade second-degree heart block, presumably as a result of high vagal tone. In anxious animals, an occasional premature supraventricular beat may also be noted.4 With the exception of sinus bradycardia and sinus tachycardia, cardiac arrhythmias and conduction disturbances are infrequently detected in camelid species. However, a variety of rhythm disturbances are occasionally observed in animals that are experiencing environmental adversity, are systemically ill, or have serious underlying congenital or acquired heart disease. Observed rhythm disturbances include premature atrial and ventricular depolarizations, supraventricular and ventricular tachycardia (SVT or VT), atrial fibrillation (AF), high-grade and complete AV block, and preexcitation.3,8
Thoracic Radiology
Thoracic radiographs should be obtained as part of the clinical evaluation whenever cardiac disease is suspected. It is, of course, always important to integrate physical examination findings, laboratory determinations (such as arterial blood gas [ABG] analysis), and thoracic imaging studies to arrive at an accurate diagnosis. Practical considerations in adult animals often limit thoracic radiographic imaging to a standing lateral view (Figure 36-2), but this single view is usually adequate to distinguish primary airway or pulmonary parenchymal disease from pulmonary compromise caused by left-sided heart failure (Figure 36-3). Thoracic radiography is also useful for identifying pleural effusion, its severity, and its likely cause. In this regard, it is complementary to thoracic ultrasonography. In neonates, thoracic radiography is particularly useful for distinguishing heart disease from the far more common pulmonary parenchymal diseases associated with immaturity or infection. It is often possible to obtain lateral and dorsoventral radiographs in young crias, and two-view studies are advisable whenever they can be accomplished without causing undue stress to the animal. Chemical restraint should also be considered to optimize the quality of the imaging study when the condition of the animal permits. This often causes less duress compared with physical restraint.
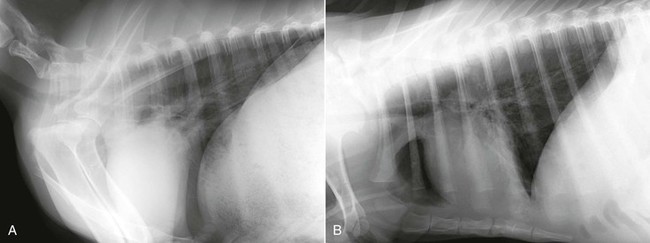
Figure 36-2 A standing lateral thoracic radiograph of a healthy mature alpaca (A) is contrasted with a radiograph of an alpaca cria obtained in lateral recumbency (B) with the forelegs pulled forward to better visualize the cranial margin of the cardiac silhouette.
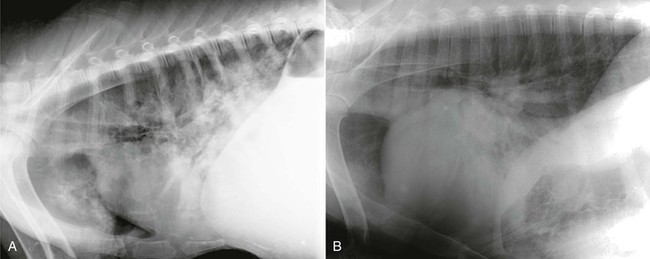
Figure 36-3 Thoracic radiography is particularly useful for distinguishing respiratory distress caused by pneumonia (A) from left-sided congestive heart failure (B). (B, From McClane M, et al: Listeria associated mural and valvular endocarditis in an alpaca, J Vet Cardiol 10:141-145, 2008.
The base of the heart is normally tilted slightly cranially in llamas and alpacas, and the cardiac long axis is normally oriented parallel to the ribs and perpendicular to the thoracic spine. The carina is typically located at the fourth rib or in the fourth intercostal space. The ratio of heart height to the height of the thorax, both measured along the cardiac long access, ranges from 0.68 to 0.74 in healthy adult llamas, and 0.65 to 0.84 in alpaca crias.8,9 Cardiomegaly is usually obvious in camelids with serious heart disease and is typically revealed by an increase in cardiac width beyond three intercostal spaces, an increase in heart height beyond three fourths the height of the thorax, and elevation of the trachea with reduction in the angle of divergence from the thoracic spine below the reported normal range of 10° to 19° (mean 14.4° + 2.0°) in adult llamas, and 9° to 22° (mean 14.2° + 3.6°) in alpaca crias.8,9 Using a modification of the Buchanan vertebral heart score (VHS) system, Mattoon and colleagues have provided scaled criteria for identifying cardiomegaly in llamas.8 According to this method, the height plus width of the normal adult llama heart ranges from 7.7 to 9.1 “vertebral lengths” (mean VHS = 8.4) or 2.75 to 3.55 times the distance from the cranial aspect of T3 to the caudal aspect of T5. In crias, the ratio of cardiac height plus width to T3-T5 has been reported as 3.12 ± 0.21, which is very similar to adult animals. Left heart enlargement is usually easily identified on the lateral radiograph, but reliable identification of right heart enlargement is often more problematic. In young crias, dorsoventral thoracic radiography allows for more sensitive detection of right heart enlargement. In crias, the mean cardiac height plus width to T3-5 ratio on dorsoventral radiography has been reported as 2.5 ± 0.25 and the cardiac to thoracic width ratio as 0.80 ± 0.06. Ambiguous radiographic findings can often be clarified by an echocardiographic evaluation, which is much more accurate for identifying specific patterns of chamber enlargement.
Echocardiography
Echocardiography is essential for the evaluation of all congenital heart diseases and most acquired heart diseases and is particularly well suited for the detection of pericardial effusion. The techniques used to perform echocardiography in camelids do not differ substantially from those used in other large animal species. Low-frequency transducers, ranging from 2 to 3.5 megahertz (MHz), are required to adequately examine adult animals. In crias, either a 5-MHz or a 7.5-MHz transducer provides the necessary resolution required for optimally imaging immature patients. Uncooperative subjects should be sedated if their clinical condition is sufficiently stable. Interrogation of the heart is typically accomplished from a combination of imaging windows located at the ventral aspect of the fourth or fifth intercostal space on the right side of the thorax and at the cardiac apex on the left. To obtain optimal images from the right side, it is very helpful to have the right forelimb positioned as far forward as the animal will accommodate so that the ultrasound transducer can be positioned as far cranial and dorsal as the lung permits. From this location, two-dimensional images should be obtained in six short-axis imaging planes through the left ventricle: (1) through the cardiac apex, (2) at the level of the papillary muscles, (3) the chordae tendineae, (4) the mitral valve, (5) the heart base at the level of the aorta and left atrium, and, if possible, (6) at the level of the main pulmonary artery as it bifurcates at the origin of the left and right pulmonary arteries (Figure 36-4). Two long-axis imaging planes should be recorded from this same location by rotating the transducer counterclockwise to obtain an image plane that optimizes a view of the left ventricular outflow tract and another imaging plane optimizing a view of all four chambers of the heart together with the mitral and tricuspid valves (Figure 36-5). Echocardiography may also be used to examine the heart of the fetus for malformations (Figure 36-6).
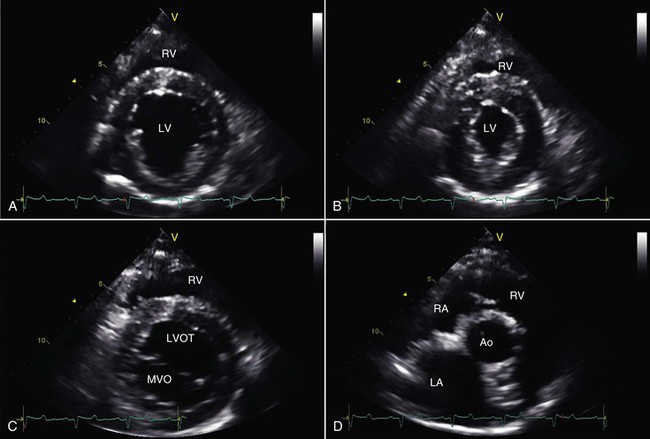
Figure 36-4 Right parasternal, short-axis, two-dimensional echocardiographic views of an adult alpaca heart at the level of the chordae tendineae are shown at end diastole (A) and end systole (B). Also shown are short-axis views at the level of the mitral valve (C) and at the heart base (D) at the level of the aorta and left atrium. Ao, Aorta; LA, left atrium; LV, left ventricle; LVOT, left ventricular outflow tract; MVO, mitral valve orifice; RA, right atrium; RV, right ventricle.
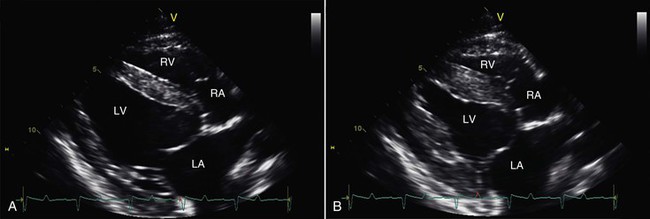
Figure 36-5 Right parasternal, long-axis, four-chamber, two-dimensional echocardiographic views of an adult alpaca heart are shown at end diastole (A) and end systole (B). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
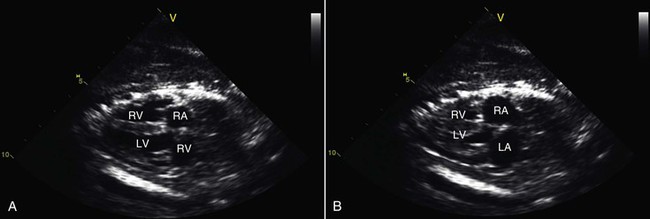
Figure 36-6 These echocardiographic images show four-chamber, two-dimensional views of an alpaca fetal heart at end diastole (A) and end systole (B). Such evaluations can be useful for establishing viability of the fetus and for identifying cardiac malformations prior to birth.
With modern echocardiography, M-mode images can be derived (postprocessed) from the digitized two-dimensional images (Figure 36-7). Alternatively, two-dimensional imaging may be used to guide the orientation of the M-mode beam to obtain the desired imaging location. Relevant measures of chamber size may be made from either the two-dimensional or M-mode images permitting the calculation of a variety of functional indices. Normal M-mode echocardiographic measures that are available are based on one study of 23 healthy llamas weighing 110 to 166 kg (mean = 138 kg) and another study of 27 healthy alpacas weighing 43 to 101 kg (mean = 68 kg).3,10 The reported normal ranges are very wide, reflecting the inclusion of animals with widely differing body weights. M-mode echocardiographic data from a small population of crias (12 alpacas and 5 llamas) were also recently made available.3 Left ventricular (LV) fractional shortening (%FS), calculated as the % change in LV short-axis diameter from end-diastole to end-systole, is the most commonly used index of systolic function. As a rule of thumb, %FS should be 25% or greater in healthy camelids, regardless of age, breed, or sex.
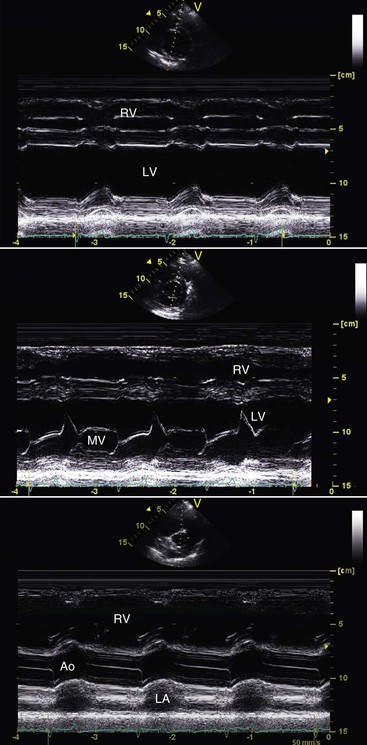
Figure 36-7 These M-mode echocardiographic scans were obtained at the level of the chordae tendineae (top), the level of the mitral valve (middle) and at the base of the heart (bottom). Ao, Aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Two-dimensional color flow Doppler imaging is a particularly useful and cost-effective imaging modality for the evaluation of camelids with congenital or acquired heart disease. Not only can the architecture of the heart be easily visualized and accurately measured, but flow disturbances within the heart and great vessels can be easily appreciated and quantified. Images are initially obtained from the right side of the thorax, where flow disturbances in the vicinity of the pulmonic, aortic, mitral, and tricuspid valves can often be appreciated. From this location, accurate flow velocity determinations via spectral Doppler can be achieved only for flow through the pulmonic valve, as such measures require the interrogating ultrasound beam to be parallel with the direction of flow. Nonetheless, it is often possible to document flow disturbances resulting from aortic, mitral, and tricuspid valvular insufficiency or from a VSD via the right parasternal long axis views (Figure 36-8). Echocardiographic imaging from the left apical region on the left side of the chest allows the generation of four-chamber and five-chamber views of the heart, the latter referring to the inclusion of the four cardiac chambers plus the LV outflow tract together with the proximal aorta. This view is particularly useful for evaluating the velocity of blood flow as it moves away from the transducer while flowing out through the aortic valve. Increased flow velocity usually indicates outflow tract obstruction, but mildly increased flow velocities may also be observed with left-to-right shunts and other causes of increased stroke volume. Quantification of valvular stenosis and insufficiency via the modality of spectral Doppler velocity recordings has supplanted cardiac catheterization as the preferred method for determining disease severity. Transvalvular flow velocities recorded in healthy llamas and alpacas are very similar to those reported for dogs and cats. It is noteworthy that often very small jets of valvular insufficiency are present in the immediate vicinity of all the cardiac valves in healthy camelids, and such observations should not be considered indicative of valve disease.11
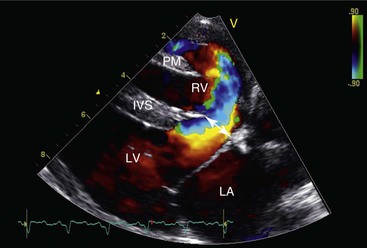
Figure 36-8 This right parasternal, long-axis, four-chamber, color flow echocardiographic image illustrates a large left-to-right shunting high ventricular septal defect (arrow) at the base of the interventricular septum (IVS). LA, Left atrium; LV, left ventricle; PM, right ventricular papillary muscle; RV, right ventricle.
Congenital Heart Defects
The most commonly reported abnormalities of the camelid cardiovascular system are congenital heart malformations. Boon and co-workers summarized the prevalence of congenital heart disease in llamas presented for evaluation at Colorado State University (24 of 663 total camelid admissions = 3.6%) and from data obtained from the Veterinary Medical Data Base from 1986 to April 1993 (35 of 2167 = 1.6%).10 Heart malformations have been reported to comprise 6.4% of all camelid congenital defects and were identified in 2.2% of camelids that were necropsied.12 It is important to be mindful that a substantial percentage of crias with congenital heart disease have more than one anatomic cardiac defect, emphasizing the need for a meticulous cardiovascular examination. Various reasons have been put forward for the perceived high prevalence of congenital heart disease in camelid populations compared with what is seen in other domestic hoofstock. The most plausible explanations focus on the proposed heritability of most congenital heart defects and the founder effect of a small gene pool for camelids in those countries where sizeable camelid herds have been built up from a relatively limited number of imported camelids, as well as bottlenecks in their native populations.
A large variety of congenital heart defects has been described in camelids, including VSD and atrial septal defect (ASD), vascular ring anomalies, endocardial cushion defects, patent ductus arteriosus (PDA), tetralogy of Fallot (ToF), pseudotruncus arteriosus, persistent truncus arteriosus, transposition of the great vessels (TGV), pulmonic stenosis, double outlet right ventricle, mitral and tricuspid valve dysplasia, and tricuspid valve atresia.3,10,12–19 Nineteen cases of congenital heart disease in llamas were identified from 1980 to 1990 at the University of California, Davis, and of these, 11 had VSDs, 4 had TGV, 2 had ASDs, 1 had ToF, and 1 had PDA (Dr. W.P. Thomas, personal communication). Fifteen cases of congenital heart disease have been identified in alpacas at the Oregon State Veterinary Teaching Hospital over the last 6 years including 4 with VSD, 4 with vascular ring anomaly, 2 with TGV, 1 with pseudotruncus arteriosus, 1 with ToF, 1 with hypoplastic left ventricle, 1 with coronary sinus to right atrial fistula, and 1 with peripheral pulmonary artery stenosis. The high prevalence of vascular ring anomaly in this last population reflects greater awareness of this disorder, and a particular interest in this condition at this institution.
Ventricular Septal Defects
VSDs are far and away the most common congenital anatomic heart defects encountered in camelids.3,10,12,14 VSDs often occur as isolated abnormalities, but they sometimes represent only one component of a more complex cardiac malformation. Accompanying congenital heart defects identified in camelids with a VSD include ASDs, endocardial cushion defects, the various components of ToF, tricuspid valve atresia, TGV, and pulmonary artery hypoplasia. Moreover, VSD may be accompanied by other congenital musculoskeletal, urogenital, and facial abnormalities such as a cleft palate.
In most affected animals, the systolic murmur of a VSD is best heard on the right cranial thorax. Often, an accompanying palpable systolic thrill exists in the same location. The intensity of the murmur is poorly predictive of the size of the lesion, as smaller defects may create greater turbulence through high pressure jets compared with larger defects with more profound hemodynamic consequences. In some affected animals, the point of maximum intensity of the murmur is located at the left heart base, but a right-sided murmur is almost always still identifiable. When the defect is located in the muscular portion of the interventricular septum, the murmur may be heard in a more caudal location on the right side of the thorax near the cardiac apex. On those occasions where aortic insufficiency develops as a consequence of an undermined aortic root, an additional diastolic murmur may be heard in association with the VSD murmur, producing a characteristic “to and fro” type of cardiac murmur. With sufficiently large defects, the volume-overloaded left heart dilates, develops mitral insufficiency, and eventually fails. In animals with very large defects, plexiform lesions develop in the pulmonary arterioles, resulting in the development of irreversible pulmonary hypertension. The resulting reversal of the shunt results in arterial desaturation, exercise intolerance, and central cyanosis. In such cases, no detectable heart murmur may be present.
When the shunt is sufficiently large, thoracic radiography may reveal changes suggestive of a VSD, including evidence of left-sided or biventricular heart enlargement in combination with pulmonary overcirculation, manifested as enlarged pulmonary arteries and veins. However, a definitive diagnosis is usually not possible without resorting to echocardiography (Figure 36-9). Color flow Doppler echocardiography is the preferred method to confirm the diagnosis, allowing visualization of the VSD and estimation of the direction and volume of the resulting shunt. Doppler echocardiography is also particularly useful for detecting pulmonary hypertension or coexisting pulmonic stenosis and for estimating right ventricular and pulmonary artery pressures. VSDs range in size from a few millimeters to the entire expanse of the septum. Most defects are between 0.5 and 1 centimeter (cm) in diameter and located in the membranous portion of the septum in an infracristal location. About one third of VSDs are located above the crista superventricularis. Less often, VSDs are found in the muscular portion of the interventricular septum, particularly near the cardiac apex. It is not uncommon to discover several muscular defects in an affected animal. Given the variability of the location of VSD in camelids, it is important to inspect the entire interventricular septum using a variety of unconventional imaging planes whenever a right-sided murmur is auscultated in a young camelid.
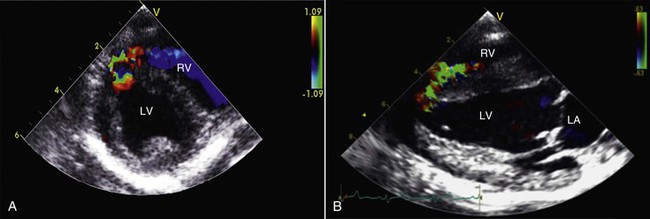
Figure 36-9 This right parasternal, short axis (A) view and accompanying long-axis, four chamber color flow echocardiographic image (B) illustrate a small left-to-right shunting muscular ventricular septal defect at the apex of the interventricular septum (IVS) in an alpaca cria. LV = left ventricle; RV = right ventricle; LA = left atrium.
Atrial Septal Defects and Patent Foramen Ovale
ASDs are far less common causes of clinical disease compared with VSDs, and anecdotal postmortem evidence suggests that they are altogether uncommon in camelids. Several different types of ASD are recognized. Ostium primum ASDs, located in the lower portion of the interatrial septum, may occur as isolated defects or as a manifestation of a complete endocardial cushion defect, sometimes referred to as an atrioventricular septal defect. Ostium secundum ASDs develop in the middle of the interatrial septum, in the region of the fossa ovalis, and are the most common type of ASD. Sinus venosus ASDs occur in the uppermost region of the interatrial septum, typically in association with anomalous pulmonary venous drainage. In crias, the foramen ovale is patent at birth and usually closes within the first 2 weeks of life. In the absence of cardiac enlargement, a patent foramen ovale (PFO) is generally regarded to be of little consequence, as the higher left atrial pressure results in physiologic closure of this potential communication between the atria. Significant shunting across a PFO only becomes problematic when another cardiac disorder causes an increase in atrial pressure and atrial enlargement sufficiently severe to allow shunting similar to that seen with a small secundum type ASD. We have also seen a 12-year-old alpaca with a PFO and bilateral CHF, in which we suspected the defect may have played a contributory role to the severity of its condition.
Congenital Valve Stenosis and Insufficiency
Semilunar valve stenosis has been reported in camelids, but such defects are uncommon. We have evaluated several alpacas with moderate isolated pulmonic valve stenosis. More often, lesions of the pulmonary outflow tract comprise one of several anatomic defects such as ToF (see “Cyanosis-Producing Defects” below). Aortic stenosis appears to be particularly uncommon in camelids. Congenital aortic and pulmonic valvular insufficiency are also apparently very rare, as is dysplasia of the mitral or tricuspid valves.17 Mitral dysplasia causes respiratory compromise and exercise intolerance when severe, whereas tricuspid defects result in jugular distention, jugular pulses, pleural effusions, and ascites. Hematocysts are sometimes observed on the AV valves at necropsy, but such lesions rarely result in valvular insufficiency. Confirmation of a suspected congenital valvular defect is best accomplished by echocardiography. No reports describing the treatment of such defects in camelids have been published.
Cyanosis-Producing Congenital Heart Defects
Complete endocardial cushion defects have been reported in a number of camelids, including a pair of llamas with a common dam.16 This malformation, sometimes referred to as an AV septal defect or AV canal, consists of lesions involving those structures formed by the endocardial cushions, including the lower portion of the atrial septum and upper portion of the ventricular septum, the septal leaflet of the tricuspid valve, and the anterior leaflet of the mitral valve. The consequence of this combination of lesions is admixture of oxygenated and unoxygenated blood within a “single chamber” compounded by the volume loads resulting from AV valvular insufficiency. A systolic murmur is typically present in such cases and may often be heard on both sides of the chest. Affected camelids usually display clinical signs within the first weeks or months of life. These signs include weakness, cyanosis, lethargy, exercise intolerance, increased time spent in recumbency, decreased nursing, open-mouthed breathing, dyspnea, and poor growth. Engorged jugular and other peripheral veins, pleural effusion, ascites, and passive congestion of the abdominal organs may be present. Echocardiography reveals the communicating heart chambers (Figure 36-10). Color flow Doppler echocardiography reveals dramatic lesions with bilateral AV valve insufficiency and shunting through the combined ASD and VSD. All chambers of the heart are enlarged, but the right side is often enlarged to the extent that it provides the apex of the heart. Severe systolic dysfunction is typically present and pulmonary hypertension may also be evident. Although one llama appears to have survived adequately for 1 year at less than 300 meters (m) above sea level, other affected camelids have shown clinical signs at a young age. Once clinical signs develop, the affected animal usually dies or is euthanized within a few weeks or months. Interestingly, one affected 2-year-old llama began to show signs only after spending a year at high altitude. Diuretics have been used to palliate the clinical signs, but they do not have much effect on the progression of the disease or the eventual outcome. The occurrence of this defect in two related camelids raises the likelihood of a genetic cause, but the heritability of this defect remains speculative.
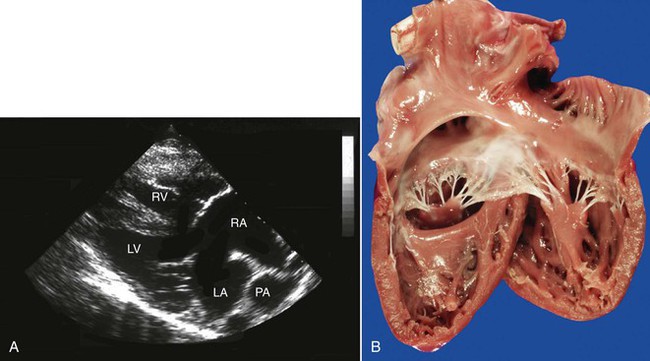
Figure 36-10 A, An echocardiogram of a large atrioventricular (AV) canal defect in a young llama. B, A necropsy specimen of another llama with complete AV canal. (B courtesy of WP Thomas.)
ToF is another well documented cyanosis-producing cardiac defect occurring in camelids and is characterized by pulmonary stenosis with hypoplasia of the pulmonary arteries, a high VSD, an enlarged and dextropositioned aorta, and right ventricular hypertrophy. This constellation of findings is the result of maldivision of the conotruncal septum during embryonic development. Extreme variants of this abnormality include those cases in which the pulmonic valve and the main pulmonary artery are completely atretic, creating the appearance of a “pseudo” truncus arteriosus (Figure 36-11). In such cases, pulmonary perfusion is accomplished by a PDA connecting the aorta to the right and left pulmonary arteries. Acyanotic forms of ToF are also seen when the pulmonic stenosis lesion is mild and right ventricular systolic pressures are not elevated above the pressure in the left ventricle. Echocardiography is particularly useful for identifying the complex anatomy of all the components of these complex congenital heart defects. Effective treatment requires surgical repair of the observed defects and, to our knowledge, this has not been accomplished in camelids.
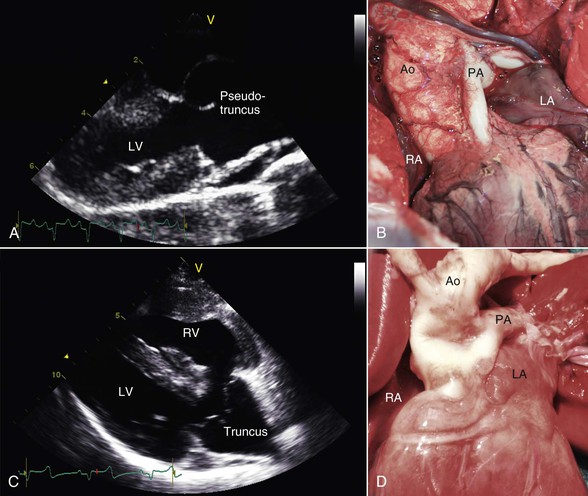
Figure 36-11 A pseudotruncus arteriosus, which is an extreme variant of tetrology of Fallot, is shown in an affected alpaca cria via echocardiography (A) and at necropsy (B). Note the close resemblance to an actual truncus arteriosus defect in a different alpaca cria, shown via echocardiography (C) and at necropsy (D). Ao, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
TGV has been documented in both llamas and alpacas on multiple occasions, yet this disorder is very uncommon in other domesticated animals. In this condition, the pulmonary artery arises from the left ventricle and the aorta arises from the right ventricle (Figures 36-12 and 36-13). This anatomic arrangement is not compatible with life unless some of the oxygenated blood returning from the lungs is able to pass from the left to the right side of the heart via an intracardiac communication or from the pulmonary artery to the aorta via a PDA. A variety of other cardiac malformations, including abnormalities of the coronary arteries, may also be identified. Affected individuals are always severely cyanotic at birth, and they usually survive only a short period. Other complex cyanosis-producing cardiac abnormalities, including hypoplasia of the left ventricle and double outlet right ventricle, have been seen in New World camelids (Figure 36-14).
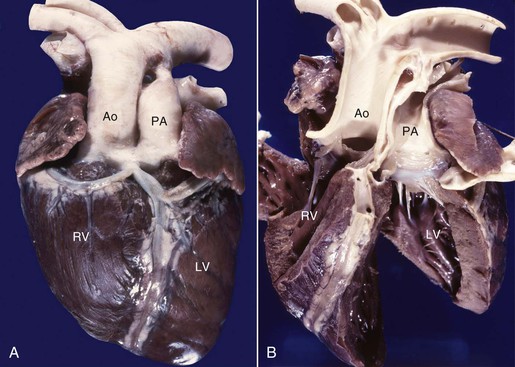
Figure 36-12 Transposition of the great vessels is shown in an affected llama, in which the aorta (Ao) arises from the right ventricle (RV) and the pulmonary artery (PA) arises from the left ventricle (LV). Note the single large coronary vessel extending down the anterior aspect of the ventricles (A), which clearly takes origin from the aortic root (B).
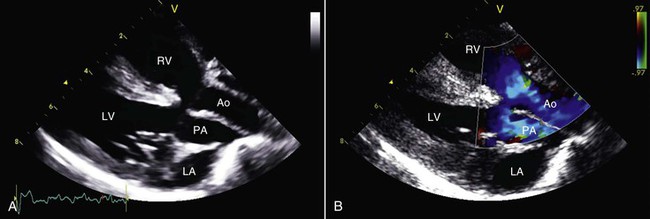
Figure 36-13 The appearance of transposition of the great vessels in an affected llama is show by two-dimensional (A) and color flow Doppler (B) echocardiography. Ao, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
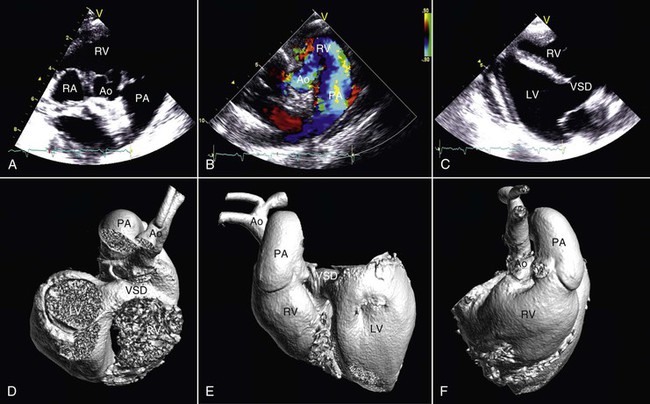
Figure 36-14 In this alpaca cria with a double-outlet right ventricle, the aorta (Ao) and pulmonary artery (PA) both arise from the right heart as shown by two-dimensional (A) and color flow Doppler (B) echocardiography. Survival is possible in the short-term because of the presence of a large ventricular septal defect (C). Views from three-dimensional casts of the heart of the same animal obtained via contrast-enhanced computed tomography (D, E, and F). Ao, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle; VSD, ventricular septal defect.
Vascular Ring Anomalies
Vascular ring anomalies have been reported in both alpacas and llamas.18,19 In contrast to other domestic species, the most common abnormality identified is a left aortic arch with either a right ligamentum arteriosum or small right PDA. This anomaly is often accompanied by aberrant origination of the right subclavian artery (Figure 36-15). The more familiar right aortic arch with constricting left ligamentum has also been observed. In those cases with a PDA, the size of the shunt was small, and a continuous murmur was only noted in one affected alpaca. Most affected animals presented at ages 3 to 5 months, and the clinical signs were largely attributable to constriction of the esophagus. Dysphagia, abnormal regurgitation, choke, bloat, and failure to thrive are common clinical signs. Cough may also occur as a consequence of tracheobronchitis or pneumonia caused by inoculation of the airways with swallowed and regurgitated food. On occasion, the development of clinical signs may be delayed until early adulthood. Routine thoracic radiography often demonstrates a dilated esophagus both cranial and caudal to the heart base, sometimes with retained ingesta. It is sometimes possible to identify a persistent right aortic arch on a dorsoventral thoracic radiograph, but other vascular ring malformations may easily escape detection.
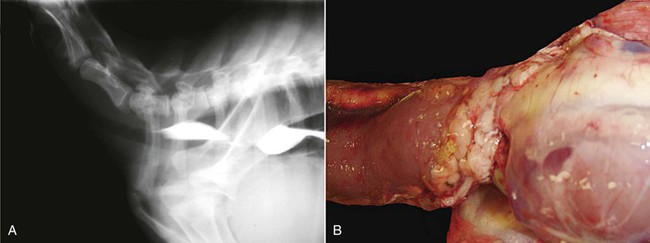
Figure 36-15 A variety of vascular ring anomalies have been reported in alpacas and llamas. Note the stricture of the esophagus in this lateral radiograph (A) obtained after barium administration. In the specimen shown in B, the vascular ring comprises a right aortic arch in combination with a left ligamentum arteriosus.
Esophagoscopy, contrast esophagography, or fluoroscopy may be performed to confirm the stenotic lesion in the esophagus at the heart base (see Figure 36-15), but visualization of the vascular malformation responsible for the constriction is best accomplished by using angiography or contrast-enhanced computed tomography (CT). Inasmuch as the surgical approach to attempt repair is dependent on the precise nature of the malformation, consideration should be given to sophisticated imaging studies whenever surgical repair is contemplated. Surgical repair has been attempted, but success has been limited, partially because of concurrent aspiration pneumonia.
Other Vascular Defects
A portosystemic shunt has been reported in one juvenile alpaca with diarrhea, poor growth, and excessive tractability for its age.20 Serum bile acid and blood ammonia concentrations were very high, and serum hepatic enzyme activities were within reference values, supportive of a diagnosis of vascular shunt. A colonic mesenteric vein portogram revealed a large extrahepatic shunt to the caudal vena cava, which was surgically ligated. The cria appeared to recover physically, behaviorally, and biochemically.
Two unrelated adult alpacas were reported to have networks of large, tortuous, anastomosing vessels in the right cranial lung lobe.21 On the basis of the predominance of tunica media over tunica adventitia, the abnormal vessels were judged to be arterial in origin. The older alpaca had severe bilateral epistaxis and pulmonary hemorrhage linked to rupture of one of these vessels. In the younger alpaca, the vascular anomaly was considered an incidental finding, with speculation that it might have become problematic with time. In humans, such lesions are often considered congenital and usually contain arteriovenous shunts.22 Approximately one quarter of the cases progress with time, whereas the majority remain static. Imaging studies were not performed on the alpacas but might have revealed the unusual vascularity. Vessel ligation or embolization or en bloc resection (lobectomy) have been used to treat this condition in other species.
Acquired Cardiac Diseases
Pericardial Disease
Pericardial disease is uncommon in llama and alpacas, and only a few reports of pericardial disease appear in the literature.3,23–25 Echocardiographic identification of pericardial effusion is the most common diagnostic test (Figure 36-16). One case of pericardial effusion causing cardiac tamponade has been reported in a 2-year-old pregnant alpaca.23 In this case, successful treatment was accomplished by pericardiocentesis and administration of antibiotics and antiinflammatory drugs. Bacteria were not cultured from the pericardial fluid in this case, and the cause of the effusion is best regarded as idiopathic. Constrictive effusive pericarditis has also been reported in a single case report of a successfully treated llama cria.24 In another successfully treated cria, pericardial effusion led to electrical alternans on ECG.25 An unrecognized septic event was thought to be the inciting factor. Others have identified pericardial effusion in association with a variety of disorders, including dilated cardiomyopathy, pleuropneumonia, pulmonary hypertension, and several different types of congenital heart disease. We have observed small effusions in a few llamas and alpacas with severe heart failure, which we considered an incidental secondary finding and of no great clinical significance. Mild effusion may occur with hypoproteinemia as well.
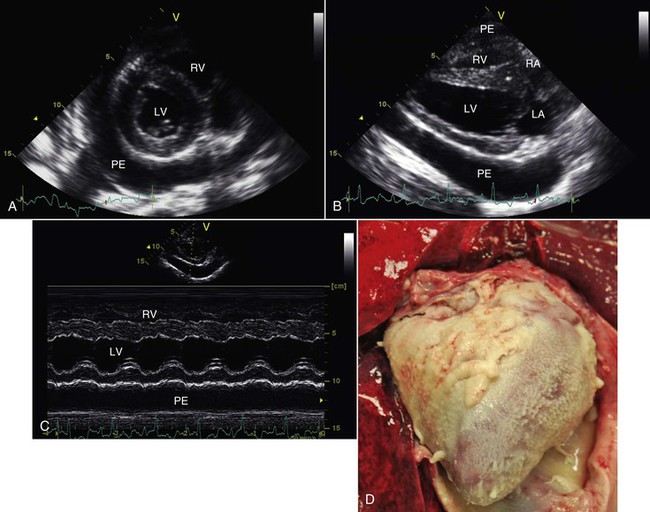
Figure 36-16 Septic pericardial effusion in this alpaca was first detected by echocardiography (A, B, and C) and later confirmed at necropsy (D). LA, Left atrium; LV, left ventricle; PE, pericardial effusion; RA, right atrium; RV, right ventricle.
Endocarditis
Endocarditis is an important but, fortunately, infrequent disorder of camelids.26–28 In most domestic animal species, endocarditis lesions are mainly confined to the cardiac valves and mural lesions are uncommon. In camelids, mural lesions within the ventricles have been observed more often and are more dramatic than valvular lesions, at least in the population of animals evaluated at Oregon State University (Figure 36-17). In our experience, the endocardial surface of the right ventricle is more commonly affected than the left, although both ventricles are affected in half the cases. Often, the leaflets of the AV valves become embedded within the thrombotic material, with the tricuspid valve slightly more commonly affected than the mitral valve. It is usually difficult to identify a remote source of infection as the primary cause of endocarditis, although preexisting abnormalities have been identified in several reports. Bacterial organisms may sometimes be recovered either via blood culture or at necropsy, but it is often not possible to recover microorganisms before or after death.
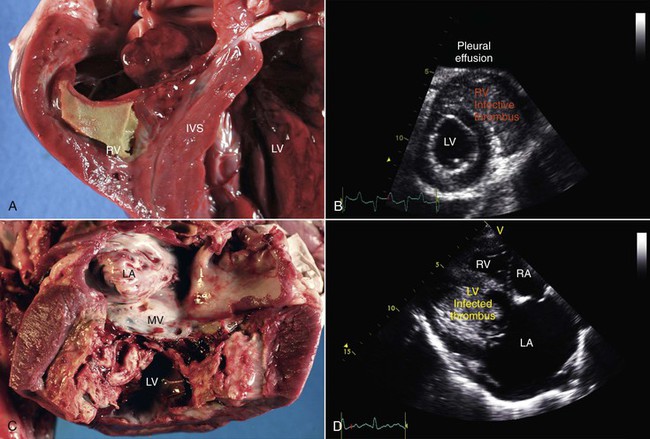
Figure 36-17 Mural endocarditis in alpacas is manifest as the deposition of infected fibrin thrombi on the walls of the right (A and B) or left ventricles (C and D), often with concurrent involvement of the ipsilateral atrioventricular valve. Note the near-obliteration of the right and left ventricular lumens noted both at necropsy (A and C) and by echocardiography (B and D).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


