Chapter 1 Disease Risk Analysis in Wildlife Health Field Studies
To calculate and manage risks better, the use of disease risk analysis has become an important tool in many areas of the veterinary sciences.21 Disease issues are often complex and predictive models, using a disease risk analysis format, may be highly effective in dealing with these disease-related challenges. Within wildlife veterinary medicine, risk analysis has also become a highly valued tool.1,2,20 Many wildlife health field studies are now directed at understanding the following: (1) diseases in wildlife populations; (2) links among wildlife, domestic animal, and human health; and (3) links between the health of captive and free-living wildlife species. Illustrative examples for each of these three areas of study include an understanding of the following: (1) the conservation implications of Batrachochytrium dendrobatidis in amphibian species; (2) tuberculosis in African wildlife and people; and (3) herpesviruses in captive and free-living elephants. Additionally, we often must make medical management decisions based on findings from wildlife health studies. For example, is vaccination a viable medical decision, or does one let nature take its course during a disease epidemic in a wild canid population? These risk management decisions may best be answered using disease risk analysis.
The growth in awareness, interests, and efforts directed at wildlife health field studies may be viewed as positive for biodiversity conservation; however, this growth is most likely the result of a significant increase in disease-related conservation challenges.8 These field studies provide a scientific process that may better direct wildlife conservation initiatives. With the current extinction crisis, limited funds for wildlife health and conservation field projects, and the zoonotic connection of diseases found in many species of conservation concern, disease risk analyses should be used to direct and perform wildlife health field studies more effectively.
Disease Risk Analysis
Risk analysis is a formal procedure for estimating the likelihood and consequences of adverse effects occurring in a specific population, taking into consideration exposure to potential hazards and the nature of their effects.23 Disciplines as diverse as economics, engineering, business, environmental science, and health all commonly apply this technique. In the health sciences, a disease risk analysis is defined as a multidisciplinary process used to evaluate existing knowledge to prioritize risks associated with the spread or occurrence of diseases.
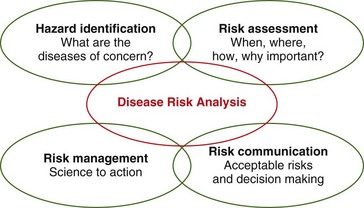
A risk analysis consists of four interconnected phases: (1) hazard identification; (2) risk assessment; (3) risk management; and (4) risk communication (Fig. 1-1). All the phases are interactive with the others—the process should not simply flow from phase 1 to phase 4 in chronologic order. A disease risk analysis is structured similar to that for other risk analyses.
Risk communication is a continuous process, necessitating respectful communication among the multiple stakeholders throughout the risk analysis.21 Risk communication should occur among field staff (those on the ground collecting data), modelers (those using data for a quantitative risk analysis), managers, laypersons, politicians, and all potentially affected parties to ensure that management policies and efforts are equitably based on the risk assessment outcome. To be of value, this requires a real-time communications network. All stakeholders must know about and understand the risks and options, with a clear statement of acceptable risk. Additionally, it must be clear as to who makes the risk management decisions. Different stakeholders often hold very different views on which risks are acceptable and who is in charge.
Hazard identification and risk assessment are sometimes grouped together because they are clearly interrelated. The criteria used to identify diseases of concern may also be used to assess the level of their associated risks. In many risk analyses, hazard identification and risk assessment are performed based solely on expert opinion or literature review. One of the most valuable products of disease risk analysis is the identification of missing data points that if obtained, would enhance a broader understanding of disease risks for a population or project. For a disease risk analysis to provide the highest quality outputs, hazard identification and risk assessments should be based on scientific data collected from the field and pertinent to the analysis in question. Providing these necessary data points for disease risk analysis are best performed by implementing standardized disease surveillance and monitoring systems.6,15,22
Outputs of a disease risk analysis may include the following: (1) a visual representation (e.g., flow charts, tables, graphs) of the analysis; (2) identification of relationships that may not have been immediately obvious; (3) identification of missing data points necessary to better understand disease risks (e.g., need for further studies); and (4) identification of critical control points to facilitate the development of cost-effective management strategies. Critical control points are any location, practice, procedure, or process at which control may be implemented over one or more factors and, if controlled, may minimize or prevent a hazard.23 Therefore, critical control points are important in the context of planning strategies that may minimize the risks of disease by identifying those actions that should be taken (e.g., risk management).
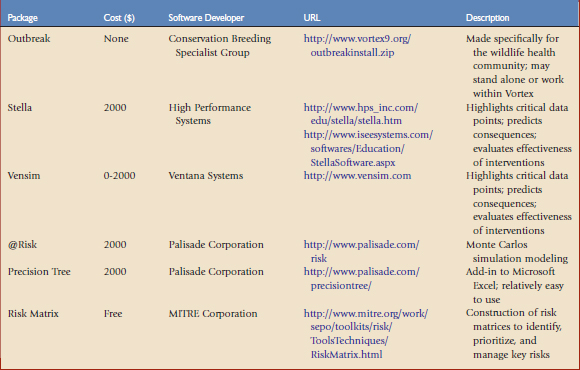
Disease risk analyses may be qualitative or quantitative. Qualitative analyses indicate the likelihood of an outcome expressed in terms such as high, medium, low, or negligible. Quantitative analyses indicate an outcome expressed numerically (e.g., there is a 10% chance that 5% of the pronghorns will develop capture myopathy). A quantitative disease risk analysis may be time-consuming and require large amounts of resources and possibly advanced training in modeling and epidemiology. Fortunately, there are a number of quantitative risk analysis software programs that go beyond deterministic models, providing stochastic capabilities (Table 1-1). In quantitative analyses, numeric values are attached to various stages of release, exposure, and consequence pathways to generate a numeric estimate of total risk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree