Chapter 13
Diagnostic Endoscopy
Before the 1990s, there were only sporadic reports of reptile endoscopy. The majority of earlier reports described the use of endoscopy to examine or retrieve foreign objects from the gastrointestinal tract, along with case descriptions of coelioscopy, bronchoscopy, and urogenital endoscopy.1–11 More recently, there has been further development and reviews of single-entry and multiple-entry techniques that have expanded endoscopic applications in the class Reptilia.12–15 In particular, validation of endoscopy procedures in lizards (e.g., liver and renal biopsy in iguanas), chelonians (e.g., renal biopsy in various species and neonate gender identification), and snakes (e.g., pulmonoscopy in Royal Pythons) have helped confirm its safety and diagnostic value.7–11,16 Given the often small and fragile nature of reptile pets, the continued development of minimally invasive endoscopy appears assured in these taxa.
Since Taylor’s original chapter on reptile endoscopy was published in the second edition,17 the 2.7-mm telescope system has continued to gain popularity as an invaluable diagnostic tool in reptile practice. Endoscopy has continued to grow and evolve with multiple journal articles published and proceedings papers presented since the last edition. Indeed, the latest reference cited in the original chapter was from 2001; therefore attempts have been made to cover the past decade of improvements and refinements in this edition. Nevertheless, most if not all of the information that appeared in the second edition is still just as relevant today, and although this is a stand-alone chapter, it would be wise to read it in conjunction with Taylor’s original chapter in the earlier edition.
Rigid Endoscopy Equipment
Despite the advances in flexible endoscopy equipment, rigid endoscopy still maintains its central role in reptile diagnostics largely because of its abilities to gain access to most areas of interest in most reptiles. However, given the variation in reptile size, species-specific anatomy, and variety of procedures that may be performed, a selection of different endoscopes and instruments is recommended, which can then be tailored to suit individual practice needs (Table 13-1). The following equipment descriptions are restricted to Karl Storz simply because that is what is known and used by the author, who receives no salary or consultancy fees from Karl Storz Endoscopy. Other manufacturers may be able to supply similar equipment.
Telescopes and Sheaths
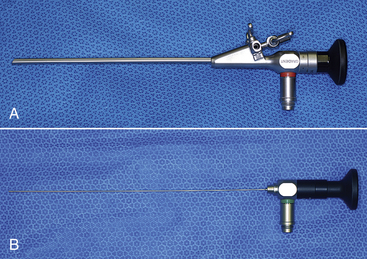
The 2.7-mm telescope system, first championed by Taylor, still remains the workhorse in exotic animal practice because of its versatility and ability to be expanded as individual practice caseload dictates.18–20 At time of press, a new 2.7 mm telescope with a significantly larger image size was released. The 2.7-mm Hopkins telescope can be used with a 3.5-mm protection sheath or with a 4.8-mm operating sheath that possesses ports for gas or fluid infusion and an operating channel for the introduction of 1.7-mm (5F) instruments (Figure 13-1). A smaller 1.9-mm telescope with an integrated sheath (3.2 mm) that can accommodate introduction of 1-mm (3F) instruments and a 1.0-mm semirigid (fiberoptic) miniature straight endoscope are particularly useful for small species (Figure 13-2).
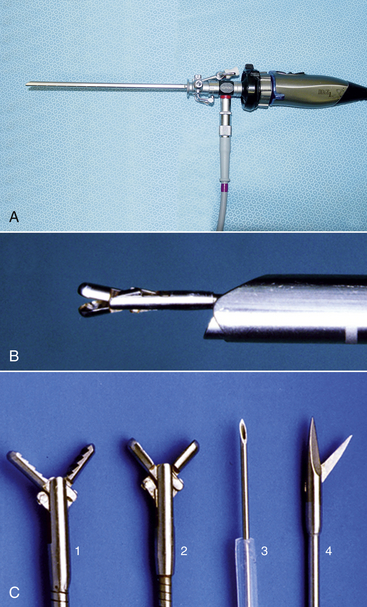
FIGURE 13-1 A 2.7-mm telescope system. A, 2.7-mm telescope housed within a 4.8-mm operating sheath, connected to a light cable and an endovideo camera. B, A 1.7-mm biopsy forceps inserted down the instrument channel and emerging directly in front of the telescope. C, A variety of 1.7-mm instruments can be used through the operating channel, including retrieval forceps (1), biopsy forceps (2), remote injection/aspiration needle (3), and single-action scissors (4). (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
Flexible Endoscopes
There are two main classifications of flexible endoscope: the fiberscope, which transmits the image via a bundle of fiberoptic fibers, and the video endoscope, which transmits the image electronically from a charge-coupled device (CCD) stationed at the distal tip. Fiberscopes are less expensive and are available in smaller diameters but have the disadvantages of pixilation and poorer image quality compared with video endoscopes (Figure 13-3). Video endoscopes tend to be larger in diameter because of the need to accommodate the terminal CCD chip, and their initial purchase costs have been considerably higher. Furthermore, until relatively recently, flexible and rigid endoscopy light sources and cameras were generally incompatible, which necessitated separate rigid and flexible tower systems. This undoubtedly forced practitioners to purchase one or the other. Fortunately, the advent of modern cameras and light sources now enables practitioners to invest in just one endoscopy tower that can be used with both rigid and flexible endoscopes.
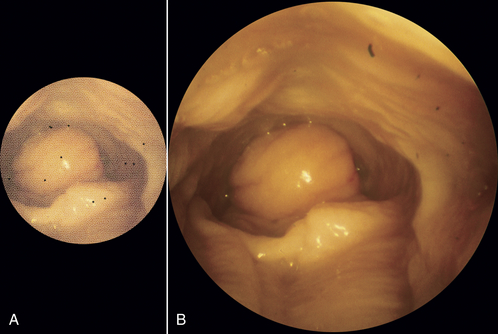
FIGURE 13-3 Relative comparison in endoscopic views of a snake’s stomach using a 2.5-mm fiberscope (A) and 5.9-mm video bronchoscope (B). Note the smaller image, increased pixilation, and black dots (broken fibers) associated with the fiberscope. (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
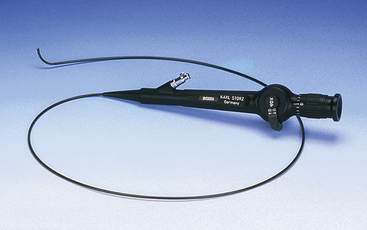
Flexible endoscopes vary from 14 mm to less than 1 mm in diameter, and those greater than 2 mm often possess a working channel (for instruments) and tip deflection. Larger flexible scopes greater than about 7.8 mm often have four-way tip deflection, working channels, and air insufflation and suction (Figure 13-4). Current technology limits the smallest video endoscope to 3.8 mm although future improvements are expected; therefore smaller fiberscopes (0.5 to 2.5 mm) maybe more useful in small reptiles. However, these smaller fiberscopes often have just one-way or two-way tip deflection and smaller working channels (Figure 13-5).

FIGURE 13-4 A 9-mm × 140-cm video gastroscope with 2.2-mm working channel attached to a xenon light source and endovideo camera. Insert, Handling technique that permits the endoscopist to control four-way tip deflection using thumb while first finger operates the two buttons that control air insufflation, suction, and fluid irrigation. (Courtesy KARL STORZ GmbH & Co. KG.)
Light Sources
The telescope is connected via a fiberoptic light guide cable to a light source (Figure 13-6, A). Halogen light sources are less expensive and are effective for small animals less than 2 kg, but xenon light sources provide better quality light and an intensity that can illuminate the body cavities of large animals. The hybrid xenon light source of the mobile Karl Storz Telepak is only 25 watts but has been used to perform coelioscopy in giant tortoises up to 100 kg.
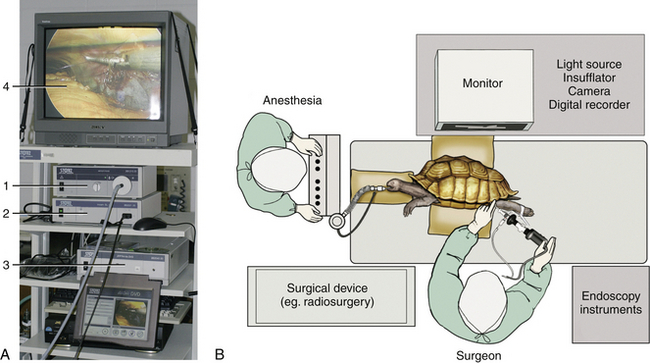
FIGURE 13-6 A, Rigid endoscopy tower with xenon light source (1), endovideo camera base unit (2), DVD digital capture device (3), and monitor display (4). (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.) B, Operating room layout demonstrating the preferred ergonomics and positioning for a right-handed surgeon performing chelonian coelioscopy. Note how the surgeon is facing the monitor and endoscopy instruments are within easy reach on the right side. (Art by Kip Carter [UGA], © University of Georgia Research Foundation, Inc.)
Cameras
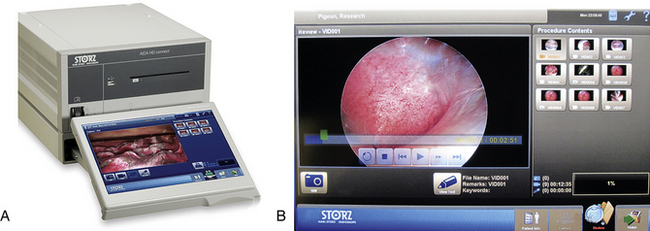
An endovideo camera connected to the eyepiece of the telescope, although once considered a luxury addition, is an integral part of the endoscopy system and greatly facilitates the surgeon’s performance (see Figure 13-6, A). Cameras, available in both European PAL and American NTSC formats, can vary dramatically in cost from budget single-chip cameras to three-chip digital high-definition (HD) models. The traditional video system provided a resolution of 720 × 480 pixels; therefore it inevitably led to some cropping of the image for display on a traditional 4:3 aspect monitor. The advent of HD systems has increased the video size to 1920 × 1080 pixels. In addition, the new 16:9 aspect ratio provides a 33% wider field of view while progressive (as opposed to interlaced) scanning produces improved resolution, color clarity, and refresh rates. Operating room setup is important: the monitor should be positioned directly in front of the endoscopist, and instruments should be within easy reach (Figure 13-6, B). In summary, the fact remains that any camera will greatly improve performance compared with just the use of an eyepiece and will also facilitate photodocumentation.
Photodocumentation
The ability to record still photographs and video of procedures is becoming increasingly important for maintaining accurate medical records, as well as for generating pictures for brochures, presentations, or scientific publications. Most camera systems generate s-video or digital outputs that can be connected to a myriad of nonprofessional, commercial recording devices (e.g., laptops and DVD recorders) that can ultimately save material in various formats to digital media. Professional-grade devices, although more expensive, provide dedicated functions that are important in medicine (Figure 13-7). For example, most professional endoscopy recording devices will support still images (TIFF, bitmap, JPEG), and video (MPEG2) with audio voiceover (WAV). Modern systems can also provide network and web-based storage and file sharing, including industry-standard Digital Imaging and Communications in Medicine (DICOM) support.
Mobile Units
The Tele Pack Vet system (Karl Storz Veterinary Endoscopy Goleta, Calif) combines a hybrid xenon light source, endovideo camera, 12-inch color liquid crystal display, with digital image and video capture and storage into a portable unit that is well-suited for veterinarians working in the field (Figure 13-8). An even more mobile option is the EndoGo camera and battery-powered light (Envisionier Medical Technologies Inc., Woodstock, Ga), which provides real “pocket-sized mobility”, with reductions in light intensity and quality (Figure 13-9).21
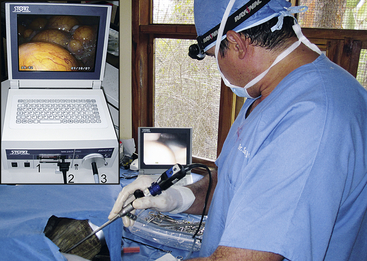
FIGURE 13-8 The Tele Pack (Karl Storz Veterinary Endoscopy, Goleta, Calif) is a mobile unit that can be readily transported and used in field situations. Shown is an endoscopist performing coelioscopy in a Galapagos Tortoise (Chelonoidis [Geochelone] nigra) at the Charles Darwin Research Station, Santa Cruz Island, Galapagos Islands. Insert, A close-up of the older Tele Pack indicating the PCMCIA card (1) for image storage, camera (2), and light source (3) connections. A more modern unit is now available that has a larger screen and both still and video capture. (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
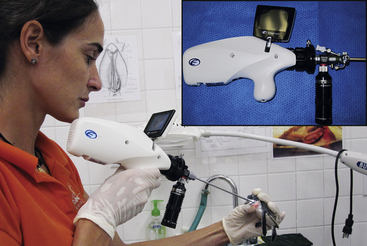
FIGURE 13-9 The EndoGo (Envisionier Medical Technologies Inc., Woodstock, Ga) is a compact, battery-powered endoscopy camera and LED light system that offers true pocket-sized mobility. In this example, the endoscopist is performing an upper gastrointestinal examination in a neonate Green Sea Turtle (Chelonia mydas) at the Grand Cayman Turtle Farm, British West Indies. Insert, Close-up of the EndoGo camera and E-Zenon LED light source attached to a 2.7-mm telescope. (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
Saline and CO2 Insufflation
Some form of insufflation is required for most coelioscopy, urogenital, or gastrointestinal procedures. For coelioscopy, medical grade CO2 gas delivered by a dedicated endoflator is preferred (Figure 13-10), but air delivered by syringe or a small aquarium air pump has been used with limited success. The risk associated with the use of air, specifically air emboli, is rare but must not be ignored. Dedicated endoflators precisely maintain the set insufflation pressure within the coelom by matching gas in-flow with any leakage.

FIGURE 13-10 Electronic endoflator designed to precisely control the delivery of CO2 for insufflation. In this example, the patient pressure, CO2 flow rate, and total gas consumption are 4 mm Hg, 0.4 L/min, and 1.3 L, respectively. (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
In some situations, the use of sterile saline can be especially helpful for creating mild distension. Saline infusion is particularly useful when dealing with a hollow viscus (e.g., gastrointestinal tract, bladder, and cloaca) or when coelioscopy is performed in small reptiles (e.g., neonate chelonian gender identification). Saline infusion may also be preferred for coelioscopy of certain aquatic reptiles in which potential ill effects of residual insufflation gas on postoperative buoyancy may occur (Figure 13-11).
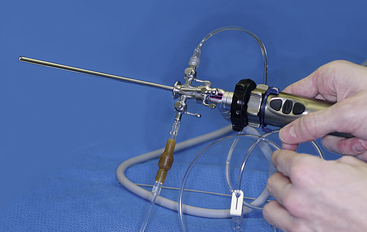
FIGURE 13-11 A simple saline infusion system requires a bag of sterile saline to be suspended above the animal (not shown) and an ingress fluid delivery line attached to one of the ports on the 4.8-mm operating sheath. A second intravenous line is used as an egress and empties into a bucket under the table. By adjusting the sheath ports, the endoscopist can control the rate of fluid entering and leaving an area. Such a system works well for neonate turtle coelioscopy, cloacoscopy, cystoscopy, and gastrointestinal evaluations. The temperature of the saline should be appropriate for the patient so that hypothermia is not induced. (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
Hand Instrumentation
Rigid Endoscopy
The basic and most frequently used instruments include biopsy forceps, retrieval forceps, scissors, and needle (Figure 13-1, C). The 1.0-mm and 1.7-mm (3F and 5F) grasping forceps are useful for manipulating tissues, debriding, and retrieving foreign objects or parasites. The fine aspiration/injection needle can be used for the aspiration of fluid from cystic structures in cases in which biopsy may be contraindicated because of postsampling leakage. The needle can also be used for irrigation and drug administration. The 1.0-mm and 1.7-mm flexible biopsy forceps are used to harvest tissue samples for histopathologic and microbiologic analysis in patients as small as 30 g. The small sample size usually permits the collection of several biopsy samples for multiple laboratory tests and serial biopsies to monitor disease progression over time for assessment of a response to treatment. To take a tissue sample, the endoscopist inserts the biopsy forceps down the operating channel and into the field of view (Figure 13-12).
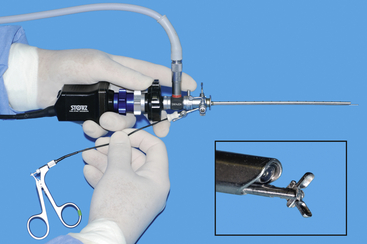
FIGURE 13-12 A 2.7-mm telescope within a 4.8-mm operating sheath with a TriCam three-chip endovideo camera and light guide cable attached (Karl Storz Veterinary Endoscopy, Goleta, Calif). Biopsy forceps (1.7 mm) passed through the working channel emerge in front of the terminal lens (insert). (© University of Georgia Research Foundation, Inc.)
It is much easier to advance and manipulate the sheath–telescope–instrument as a single device than to try and keep the sheath–telescope still and independently move the biopsy forceps back and forth. With the biopsy forceps held open, the sheath–telescope–instrument is advanced to the tissue of interest, and when tissue enters the biopsy cup, the handle is released. These instruments are delicate, and the biopsy handle is often only required to open the biopsy jaws. The handle’s spring mechanism is usually sufficient to take a soft tissue biopsy sample without additional manual pressure, as long as the instrument is sharp. Clamping down on the handle will damage the forceps and increase biopsy crush artifact.
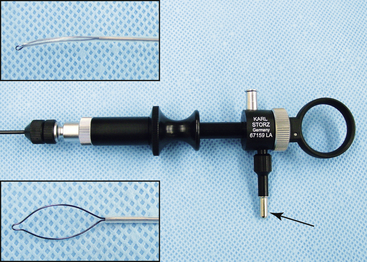
Some organs may be protected by a more fibrous membrane. The fixed blade of the scissors is inserted at a shallow angle through the membrane, and the sheath–telescope–scissors are advanced as a single unit, cutting the membrane as they proceed. The scissors can then be replaced by the biopsy forceps to take a sample through the capsular incision. Other instruments that can be invaluable in specific situations include a wire basket retrieval device (for removal of fibrous foreign bodies or stones; Figure 13-13), a polypectomy snare (for removal of small growths and polyps; Figure 13-14), and a radiosurgical needle electrode (for pinpoint ablation or coagulation).
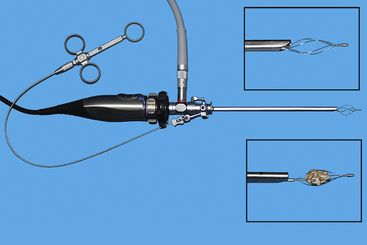
FIGURE 13-13 A 2.7-mm telescope within a 4.8-mm operating sheath with Image-1 endovideo camera and light guide cable attached. Wire-basket retrieval device (1.7 mm) passed through the working channel with close-up views of the device opened and closed around a stone (inserts). (© University of Georgia Research Foundation, Inc.)
Equipment Cleaning, Sterilization, and Storage
Equipment should be stored in a safe and secure manner, and a variety of metal and plastic containers have been designed to purposefully house items and withstand sterilization (Figure 13-15). It can be a disadvantage to store equipment sterilized, as this may dissuade the novice endoscopist from reaching for the endoscope to practice on cadavers.
FIGURE 13-15 Dedicated storage and sterilization trays (A and B) help to protect equipment. Note also the protective plastic sheaths (arrows) over the fine diameter telescopes in (A). (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
Rigid Telescope and Instrument Handling
The fact that most pet reptiles weigh less than 2 kg requires careful control of the telescope; the eyepiece and camera should be supported with the superior hand, and the distal shaft of the telescope should be held by thumb and forefinger of the inferior hand (Figure 13-16, A). Handling the telescope in this fashion provides fine control without tremors; however, for a single surgeon to be able to use instruments, a modified hold is required. Inserting and manipulating instruments through the operating sheath requires changing from a two-handed to a one-handed technique. The usual thumb and forefinger support of the telescope shaft is now adjusted so that the inferior hand takes the entire weight of the sheath–telescope–camera system. The thumb is slid up the shaft of the sheath, and the fingers are curled over the top to encircle the sheath. Now that the sheath is grasped in a fistlike grip and the sheath is further supported by the thumb to prevent rotation, the superior hand can be removed to pick up and insert an instrument down the operating channel (Figure 13-16, B). This can only be performed with a correctly sheathed telescope; damage will occur otherwise.
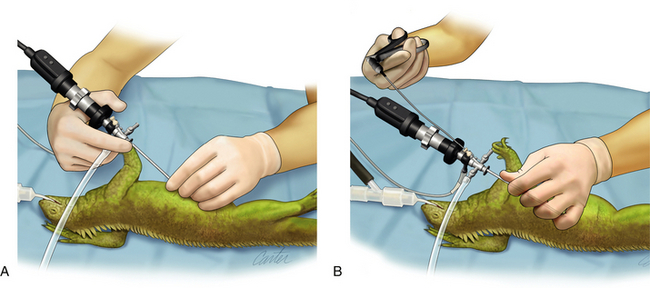
FIGURE 13-16 Correct handling of the 2.7-mm telescope housed within the 4.8-mm operating sheath. A, The two-handed technique for general evaluation involves supporting the sheathed telescope and camera with the superior hand while the thumb and forefinger of the inferior hand provide fine motor control and prevents tremor. B, The one-handed technique facilitates instrument use by a single surgeon and involves the inferior hand taking the weight of the sheathed telescope and camera by gripping the entire shaft, with the thumb slid up the sheath to prevent rotation. The inferior hand is now free to manipulate an instrument into the operating channel. (Art by Kip Carter [UGA], © University of Georgia Research Foundation, Inc.)
Biopsy Care and Handling
It is interesting to note that various studies have concluded that endoscopic biopsy specimens are more diagnostic than ultrasound-guided aspirates or biopsy specimens.22–24 Endoscopic biopsy specimens are delicate and measure approximately 1 mm3 when collected with a 1.7-mm forceps. Handling and other histologic artifacts are reduced by gently dislodging the tissue from the biopsy cups into a small volume of sterile saline, then decanting into a biopsy cassette or bag, and submitting in 10% neutral buffered formalin (Figure 13-17, A).11 Picking the biopsy specimen from the instrument with a needle will cause severe damage, and even moistened cotton-tipped applicators have been shown to cause tissue alteration (Figure 13-17, B, C). Biopsy samples for microbiologic analysis are best submitted in sterile saline for immediate processing. Alternatively, if mailed to a laboratory, they should be submitted in appropriate transport media. For the submission of samples for toxicologic or parasitologic analysis, it is wise to consult with the laboratory before sample collection.
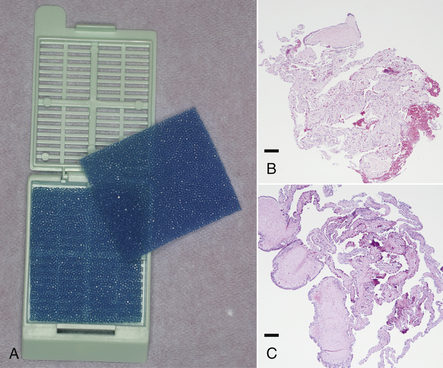
FIGURE 13-17 A, Endoscopic biopsy specimens are best sandwiched between foam and secured within a histology cassette. B, Crush artifact in a snake lung biopsy sample caused by a cotton-tipped applicator used to transfer the tissue from forceps to histology cassette. C, Another snake lung biopsy sample exhibiting reduced artifact because it was shaken from the forceps into sterile saline before being decanted into the histology cassette. (Courtesy Dr. Stephen J. Divers, University of Georgia, Athens, Ga.)
Preoperative Evaluations and Anesthesia
Patient Selection, Evaluation, and Contraindications
Knowledge of species-specific anatomy, physiology, husbandry, and nutritional requirements are vital for properly evaluating the management and medical history of a reptile. Inexperienced clinicians are directed to reviews on the subject and should prepare ahead of time.25,26 Complete physical examination, including accurate body weight, is essential but may require sedation or anesthesia for aggressive snakes or uncooperative animals, particularly chelonians. Serial clinicopathologic data can be helpful in quantifying dehydration or indicating infection/inflammation, organ damage, or dysfunction. However, most published reference ranges are too broad to be of value unless patient data are severely abnormal. Complete blood counts and biochemistry panels (i.e., total levels of protein, albumin, globulin, aspartate transaminase, bile acids, glucose, creatinine phosphokinase, calcium, phosphorus, sodium, potassium, chloride, uric acid, ammonia, and urea for aquatic species) are recommended, and even in animals less than 100 g, hematocrit, a blood smear for differential white cell counts, and a total protein level can still be obtained.
Patient Preparation and Anesthesia
Fluid therapy is the mainstay of stabilization, and hydration with crystalloids (with an osmolarity of 260 to 290 mOsm/L) at 25 to 45 mL/kg/day is recommended.27 Fluids may be given intravenously or intraosseously for critical cases, or intracoelomically, subcutaneously, or orally. It is advisable to avoid oral or intracoelomic fluids immediately before gastroscopy or coelioscopy.
Certain examinations (e.g., stomatoscopy and cloacoscopy) may be possible in the conscious or sedated patient with the use of a mouth gag and appropriate restraint, but complete immobilization is preferred so that damage to equipment, patient, or staff is avoided. General anesthesia is recommended for all endoscopy procedures (including oral and cloacal examinations) and is required for any invasive procedures (including coelioscopy).9,28 Conscious coelioscopic examinations may be acceptable in research or wildlife under an appropriate experimental license, but in clinical practice, analgesia and anesthesia are considered mandatory. Some authors have reported using only local anesthesia for chelonian coelioscopy, but this likely provides inadequate analgesia for more involved coelioscopic procedures.29 In a recent comparison of local versus general anesthesia for chelonian coelioscopy, anesthetic scores were significantly better for procedures conducted under general anesthesia compared with local lidocaine alone.9 A detailed description of anesthesia is beyond the scope of this chapter, but careful consideration should be given to intubation and adequate ventilation, especially when the coelom is insufflated during coelioscopy. There appear to be taxa-specific effects of opiates in reptiles given morphine (1.5 mg/kg) or hydromorphone (0.5 mg/kg): the dose seems effective in chelonians, whereas very high doses of morphine or butorphanol appear to be required for some lizards and snakes.30,31 The nonsteroidal antiinflammatory analgesic meloxicam (0.2 mg/kg subcutaneously, intramuscularly, or intravenously) is used routinely in all species and may be repeated after 48 to 72 hours, if necessary.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree