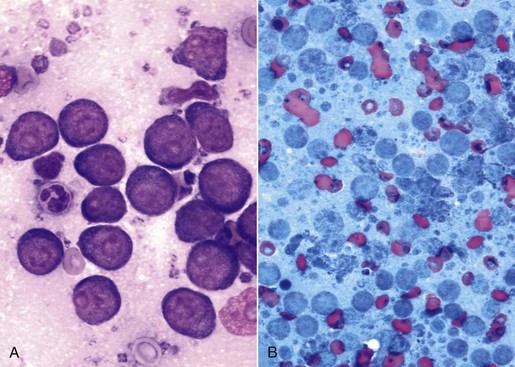
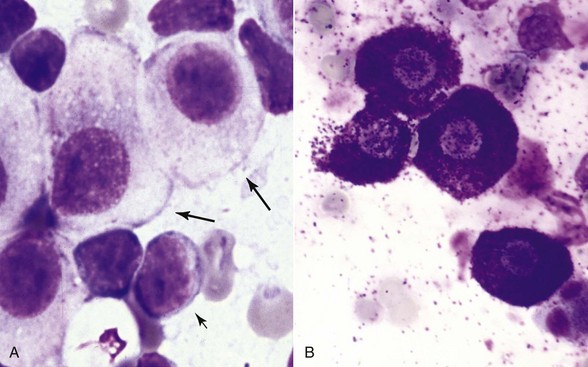
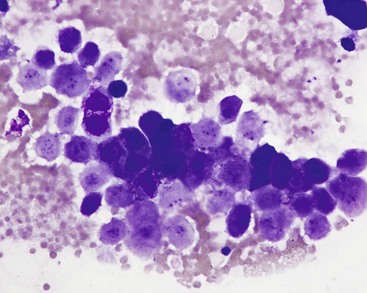
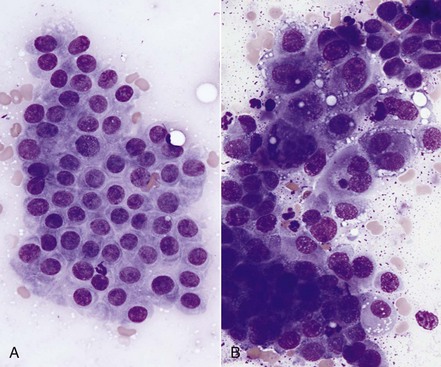
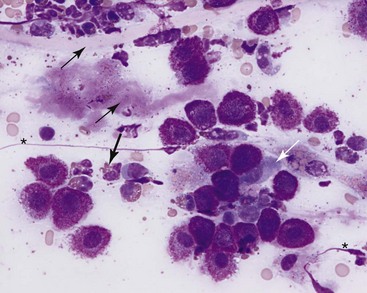
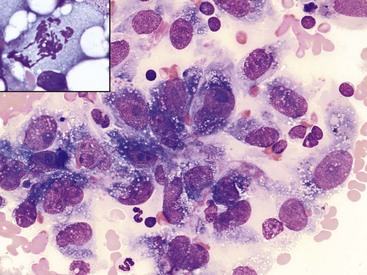
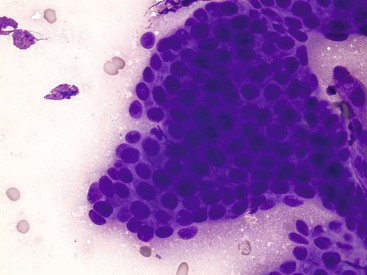
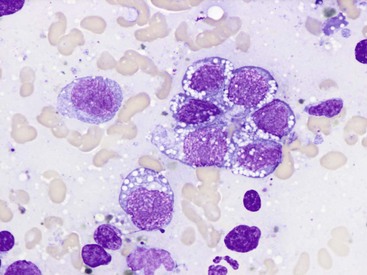
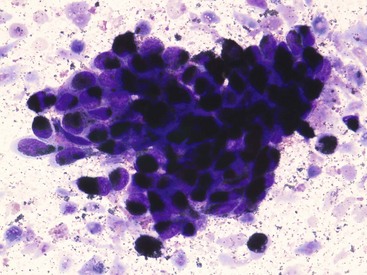
7 Proper collection and preparation techniques are prerequisites to obtaining diagnostic samples of high quality. Supplies necessary for collecting cytologic samples from a variety of tissues, body cavities, and mucosal surfaces are available in most clinics. These include hypodermic needles and syringes, scalpel blades and handles, propylene urinary catheters, bone marrow aspiration needles, cotton swabs, clean glass slides, marking pencils, and collection vials and tubes (tubes with ethylenediaminetetraacetic acid [EDTA] and plain sterile tubes). For aspiration of internal lesions, obtained by guidance with ultrasonography or computed tomography (CT), longer spinal needles and butterfly catheters (used to connect the spinal needle to the aspirating syringe) are useful. Cytologic specimens also can be made from tissues collected during biopsy (see Chapter 9). All supplies should be assembled in one location for ready access. Although life-threatening situations are rarely encountered when collecting cytologic specimens, supplies and medications should be available to control bleeding and to treat anaphylaxis. The latter occurs rarely when aspirating mast cell tumors because of release of histamine. Fine-needle aspiration (FNA) is by far the most common method for collecting cytologic specimens. Small-gauge needles (22 to 25 g) are sufficient for smaller lesions and result in less hemorrhage. Large-gauge needles (18 to 20 g) may be required to collect sufficient material from masses containing abundant matrix (i.e., firm masses and sarcomas), but specimens may contain more blood. Medium-sized syringes (12 to 15 cc) yield more vacuum for aspiration than smaller syringes (3 to 6 cc). The intent of aspiration is to draw cells into the needle shaft, not to fill the syringe with material unless the lesion is fluid-filled. After the needle is inserted into the lesion, vacuum is maintained in the syringe while the needle is redirected into the tissue several times to collect a broad representation of cells. This is especially important when aspirating lymph nodes to search for metastasis. Following aspiration, vacuum is released prior to removing the needle from the tissue, the needle is removed from the tissue and then from the syringe, the syringe is filled with air and reattached to the needle, and the cells are expelled onto a glass slide. An alternative technique is to obtain cells without aspiration by holding the needle by the hub between the thumb and middle finger while covering the hub opening with the forefinger (to prevent blood or other fluids from escaping) and rapidly and repeatedly inserting the needle into the lesion with redirection until cells are packed into the needle shaft.1 This method often yields as much cellular material as the aspiration technique and produces less hemorrhage and patient discomfort. Similar to the aspiration technique, a syringe is used to expel the material in the needle onto a glass slide. A second clean slide is then placed on top of the sample and the two slides are pulled apart in parallel, taking care not to exert pressure on the sample. The aim is to obtain a monolayer of intact cells. Failure to spread the specimen immediately leads to a sample that is too thick to interpret; conversely, aggressive pressure on the sample may rupture many if not all cells, also leading to a nondiagnostic specimen. A variety of quick stains are available for immediate examination of cytologic specimens and include quick Romanowsky stains, such as Diff-Quik. A specific set of staining jars should be kept exclusively for cytologic specimens and not used concurrently for dermatologic specimens. The jars containing the stain components should be capped between uses to prevent evaporation and contamination of the fixative and stains. Maintenance, including scheduled replacement of stain components, is important to avoid artifacts such as stain precipitate and contamination with organisms or debris that might be misinterpreted. Slides should be completely air-dried prior to fixation in the methanol fixative. Stains must thoroughly penetrate the smear, and in well-stained smears nuclei should be purple (Figure 7-1, A). A thick sample requires more contact time with the stains; understained slides (Figure 7-1, B) can be restained for a longer period of time, and overstained slides can be destained with methanol and restained for a shorter period of time. If the slides will be sent to an outside diagnostic laboratory, clinicians are encouraged to stain a slide to ensure that sufficient material was collected and that cells are intact prior to submitting additional unstained slides for evaluation. Additional specimens should be collected if only noncellular material is present or if all the cells are lysed. For some lesions, the first slide prepared may be the only slide that contains cellular material. It is best to send this slide unstained to the diagnostic laboratory, but, if it is stained, be sure to include it with the other slides. Quick Romanowsky stains provide good nuclear detail and usually sufficient cytoplasmic detail for cytologic interpretation. Mast cell granules occasionally fail to stain with aqueous quick stains (Figure 7-2, A). Wright-Giemsa and modified Wright stains provide a broader palette of colors and excellent staining of cytoplasmic granules (Figure 7-2, B) but require more steps and longer staining times or use of an automated stainer. Fixation of wet smears is required for Papanicolaou staining, which is not frequently used in veterinary cytology. Heat fixation is not required or recommended for cytologic specimens. Cytochemical and immunocytochemical staining may be necessary to determine the specific tumor type. A complete list of available special stains and antibodies is beyond the scope of this chapter; consultation with a veterinary cytopathologist is recommended when considering the necessity and use of these stains. Cytochemical stains identify specific chemical compounds or structures within the cytoplasm or nucleus and include stains such as Prussian blue for iron, periodic acid-Schiff (PAS) for carbohydrates, alkaline phosphatase for identifying osteoblasts,2 and a wide variety of leukocyte markers, including Sudan black B, peroxidase, chloracetate esterase, and nonspecific esterases. Immunocytochemical staining procedures utilize antibodies to identify specific proteins or peptides within or on the surface of the cells. Common antibodies used in veterinary oncology include those directed against CD3 (T-cells), CD79a and CD20 (B-cells), cytokeratin (epithelial cells), vimentin (mesenchymal cells), and Melan A (melanocytes). Use of a single stain or antibody is discouraged, as cell lineage is rarely identified and aberrant expression by neoplastic cells may lead to an erroneous diagnosis if a single marker is evaluated; a panel of stains or antibodies usually is necessary for complete identification. What constitutes adequate cellularity depends on the type of tumor. Aspirates of mesenchymal tumors, which often contain extracellular matrix, tend to be less cellular than those of epithelial and discrete round cell tumors. The degree of cellularity also has an impact on the level of confidence expressed in the interpretation, and diagnostic opinions are often qualified with “possible” or “probable” for poorly cellular specimens compared with “diagnostic for” or “consistent with” for highly cellular specimens. All cytologic specimens contain some ruptured cells, but to render a meaningful interpretation the majority of cells should be intact. Material from ruptured cells is recognized as stringy strands of chromatin or swollen magenta nuclei, often with obvious nucleoli, and free cytoplasmic fragments (see Figure 7-1, A). Large lymphocytes and cells from endocrine tumors are highly susceptible to lysis, and extra care should be taken not to exert pressure on the cells when preparing cytologic specimens from these lesions. The submandibular salivary gland occasionally is aspirated instead of the mandibular lymph node and is recognized by clusters of foamy cells in a background of mucin and blood. When tissue containing a metastatic tumor is aspirated, the specimen may contain only neoplastic cells or may contain normal cells from the tissue (e.g., lymphoid populations in a lymph node), which helps confirm location of the tumor. Necrosis can be found in tumors that have outgrown their blood supply. Necrotic cells lack detail and consist of gray-pink, indistinct cytoplasm and amorphous nuclei (Figure 7-3); they should not be confused with apoptotic or pyknotic cells, which retain distinct cytoplasmic borders that surround condensed nuclear fragments. Determination of the number of cells exfoliated and the shape and arrangement of cells early in the cytologic evaluation aids in formulating an initial list of differential diagnoses, permitting placement of tumors in three broad categories: epithelial, mesenchymal, and discrete round cell tumors. Briefly, cells from epithelial tumors exfoliate well and are round, cuboidal, columnar, or polygonal cells arranged in cohesive sheets or clusters; cells from mesenchymal tumors exfoliate poorly and are spindle-shaped, stellate, or oval cells arranged individually or in noncohesive aggregates; and cells from discrete round cell tumors exfoliate well and are individualized round cells that are arranged in a monolayer. Cellular arrangements observed in cytologic specimens and their associated histologic correlates and tissue types have been described.3 Proper terminology should be used to succinctly describe cell populations and convey important information. The terms homogeneous and heterogeneous describe cell populations (Figures 7-4 and 7-5). Homogeneous denotes a population of one cell type (excluding erythrocytes and associated leukocytes), which is typical of most tumors. Heterogeneous refers to mixed populations of cells, which are commonly found in aspirates of inflammatory lesions; however, some neoplasms will contain heterogeneous populations of cells (e.g., mast cell tumors accompanied by eosinophils and fibroblasts [see Figure 7-5] and squamous cell carcinomas with associated neutrophilic inflammation [see later]). The terms monomorphic and pleomorphic describe the morphologic appearance of cells within a single population. Monomorphic describes cells of a single lineage in which the cells have a uniform morphologic appearance (see Figure 7-4, A). Monomorphic features typically are associated with benign tumors, but a number of malignant tumors are cytologically monomorphic. In contrast, pleomorphic is used to describe cells of a single lineage that have variable morphologic features (see Figure 7-4, B). Pleomorphic features comprise a set of criteria of malignancy and suggest malignant behavior, but can be observed in nonneoplastic cells found in primary inflammatory lesions. Criteria of malignancy are cellular features within a single population that suggest malignant behavior, with greater emphasis placed on nuclear criteria. The more criteria observed, the more likely the tumor is malignant. Cellular and cytoplasmic criteria of malignancy include variation in cell size (anisocytosis), abnormal cellular arrangement (3-dimensional [3D] clusters instead of a monolayer), cells that are smaller or larger than their normal counterpart, variable nuclear-to-cytoplasmic (N : C) ratios or N : C ratios that differ from what is expected for the cell type, intensely basophilic cytoplasm (hyperchromasia), abnormal vacuolation or granulation, and aberrant phagocytic activity. The nucleus is the most important component of the cell when determining the biologic behavior of a neoplasm. Nuclear criteria of malignancy include variation in nuclear size (anisokaryosis), unusual nuclear shape, multinuclearity, variation in nuclear size within the same multinucleated cell, nuclear fragments, multiple nucleoli that vary in size and shape within the same nucleus or among cells, increased mitoses, and nonsymmetric mitoses (Figure 7-6). When Papanicolaou stain is used, additional nuclear features such as irregular thickening of the nuclear membrane can be evaluated. Cellular gigantism (cell >10 times the diameter of an erythrocyte) and the presence of macronuclei (>5 times the diameter of an erythrocyte) or macronucleoli (larger than an erythrocyte) are particularly disturbing criteria of malignancy. In nonneoplastic cells the chromatin pattern is finely stippled in replicating or metabolically active cells and condensed in mature quiescent cells. Finely stippled chromatin is also common in rapidly proliferating neoplastic cells, and chromatin that is irregularly clumped or ropy is unusual and suggestive of a neoplastic process. Some nonneoplastic cells, including mesothelial cells, fibroblasts, and squamous epithelial cells, may have criteria of malignancy when they are highly proliferative in the presence of inflammation. Conversely, some malignant tumors such as apocrine gland tumors of the anal sac have few criteria of malignancy. Confidence in cytologic interpretation is based on the quality of the specimen, the completeness of the clinical information provided, and the experience of the cytopathologist. Terms that express the degree of certainty, such as “consistent with,” “diagnostic for,” “cannot rule out,” “probable,” and “possible,” may be used and interpreted differently by cytopathologists and clinicians, respectively.4 If the certainty of an interpretation or diagnosis is unclear, the clinician should consult the cytopathologist. Correlations between cytologic and histologic interpretations or diagnoses are highly variable, depending on tissue types, disease processes, and methods of collection and preparation. Tumors derived from epithelial tissue comprise the largest category of neoplasms and include tumors of epithelial surfaces, such as the skin and respiratory, gastrointestinal, and urogenital tracts, as well as tumors of glands and organs. Given their diverse origin, the cytomorphologic appearance of these neoplasms can be highly variable; however, some features are shared by most epithelial tumors. Epithelial cells have intercellular junctions that connect the cells to each other and do not elaborate extracellular matrix. Therefore the cells exfoliate well, resulting in highly cellular specimens, and are arranged in cohesive sheets or clusters in cytologic smears (Figure 7-7). The cytoplasmic borders of individual cells typically are distinct, but this can vary in certain types of tumors. Poorly differentiated epithelial tumors have few or no identifying features and tend to be round cells with moderate-to-high N : C ratios and basophilic cytoplasm. In some cases, the cells no longer have intercellular junctions and appear as discrete round cells (Figure 7-8). Determining the tissue of origin in these cases is difficult, and histologic evaluation, with or without immunohistochemical analysis, is necessary to define the specific tumor type. Differentiating among adnexal tumors of skin by cytologic evaluation may be difficult when identifying features are absent or when multiple cell types are present. Many adnexal tumors have a large component of basilar cells that are small cuboidal or round cells with high N : C ratios and are arranged in tightly cohesive sheets or in palisading rows (see Figure 7-7). Nuclei are uniformly round with condensed to reticular chromatin, and nucleoli are indistinct or appear as a small single nucleolus. The cytoplasm is lightly basophilic and may contain black melanin granules (Figure 7-9). Tumors of hair follicle origin and matrical cysts often have a central cystic space filled with mature squamous cells, keratin flakes, or keratin debris, and this material may be aspirated when the mass is sampled. Tumors with sebaceous differentiation contain clusters of large round cells filled with oily-appearing vacuoles that partially obscure the small central nucleus (Figure 7-10). Tumors of basal cell origin (cutaneous basilar epithelial neoplasms) include trichoblastoma, pilomatricoma, basal cell epithelioma, sebaceous epithelioma, and others, and histologic examination is usually required to identify the specific type. Fortunately, the majority of adnexal tumors are benign; malignant types can occur and typically have pleomorphic features that predict their biologic behavior.
Diagnostic Cytopathology in Clinical Oncology
Sample Collection
Collection Techniques
Cytologic Stains
Specimens of Diagnostic Quality
Nonneoplastic Cells and Noncellular Material Found in Cytologic Specimens
Description of Neoplastic Populations
Interpretation of Cytologic Specimens
Epithelial, Mesenchymal, and Discrete Round Cell Tumors
Tumors of Epithelial Tissues
Tumors of Hair Follicles and Sebaceous Glands
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diagnostic Cytopathology in Clinical Oncology