Diagnosis of Pregnancy and Evaluation of High-Risk Pregnancy
Diagnosis of Pregnancy
Depending on the method used, pregnancy evaluation may be performed as early as 2 weeks after mating. However, pregnancy loss is common in camelids, and not all females diagnosed to be pregnant during early examination will deliver a live cria. Pregnancy loss occurs in 10% to 50% of the females before 60 days of gestation.1,2 Therefore, a second pregnancy evaluation is recommended 60 days after mating to confirm pregnancy status and allow prompt rebreeding of those females that had undergone pregnancy loss. Pregnancy loss later in gestation is less common but may still occur in 3% to 17% of the females.2,3 Therefore, a third examination is recommended during the last trimester of gestation. Evaluation of the female is indicated anytime she shows receptivity to the male after a positive pregnancy diagnosis.
Sexual Behavior
Camelids are induced ovulators, and ovulation is induced by mating. In nonmated females, follicular waves emerge at regular intervals without interruption by spontaneous ovulation and luteal development. In the absence of a functional corpus luteum (CL), female llamas and alpacas are sexually receptive regardless of follicular size or estrogen concentration.4,5 The sexually receptive female assumes the copulation position of sternal recumbency when approached by the male. Nonreceptivity, manifested as spitting at the male and attempting to escape, occurs in the face of high blood progesterone concentration (≥2 nanograms per milliliter [ng/mL], or 6.4 nanomoles per liter [nmol/L]).1
In mated females, ovulation occurs 24 to 48 hours after mating. However, blood progesterone concentration does not increase significantly until 4 days after mating. During these 4 days, some females may continue to be sexually receptive. Blood progesterone peaks at 8 days and decreases thereafter to reach basal concentrations 11 to 13 days after mating.6,7 If conception has occurred, luteolysis is prevented, and blood progesterone concentration remains elevated throughout gestation.8,9 Therefore, failure to display sexual receptivity 11 to 13 days after mating indicates maintenance of a functional CL and suggests pregnancy.1,10
Behavior tests are economical and do not require special equipment or skills. However, interpretation of sexual behavior is subjective and prone to error. Accuracy of diagnosis of pregnancy based on behavior is 83% to 95%.1,10,11 Some pathologic conditions such as luteal ovarian cysts, pregnancy loss after maternal recognition of pregnancy, and abnormalities of the tubular genitalia may result in a prolonged luteal phase, leading to false-positive results. Dominant females may be reluctant to cush, especially if exposed to a young inexperienced male. Exposing a timid female to an aggressive male may result in submission to mating and mask a nonreceptive behavior in a pregnant female. The behavior test does not assess embryonic or fetal number, development, or well-being.
Progesterone
Blood progesterone concentration reflects the presence or absence of functional luteal tissue. Since luteal progesterone is required to maintain pregnancy in camelids, blood progesterone concentration remains higher than 2 ng/mL throughout pregnancy until the last 2 weeks before parturition.9 If the female has failed to conceive, blood progesterone concentration remains elevated until 11 to 13 days after mating when it reaches basal values again following luteolysis. Therefore, blood progesterone concentrations greater than 2 ng/mL 11 to 13 days after mating suggest pregnancy, whereas values lower than 2 ng/mL indicate absence of pregnancy.6,7
Depending on the reference values established by each laboratory, values between 1 and 2 ng/mL are doubtful, and the test should be repeated. Radioimmunoassay, chemiluminescence, and enzyme immunoassay are standard analytical methods for determination of progesterone concentration. No stall-side progesterone tests have been developed for use in camelids. However, a semiquantitative stall-side equine progesterone test (Target equine progesterone kit, BioMetallics, Princeton, NJ) has provided a more reliable interpretation compared with radioimmunodiffusion.12 Alternatively, concentration of progesterone or its metabolites may be evaluated in feces or milk.13
False positive diagnoses may occur in 15% of females as a result of spontaneous ovulations, luteal ovarian cysts, pregnancy loss after maternal recognition of pregnancy, and abnormalities of the tubular genitalia that may result in a prolonged luteal phase.7 A progesterone test does not assess embryonic or fetal number, development, or well-being.2,14
Estrone Sulfate
Estrone sulfate concentration may be measured in plasma or urine. A first peak in estrone sulfate concentration occurs between 21 and 27 days after mating, with concentrations being higher in pregnant llamas (44.5 ± 5.4 ng/mL) and alpacas (48.1 ± 3.8 ng/mL) than in nonpregnant animals (<1 ng/mL).8 The source of estrone sulfate during this period is thought to be the trophoblast. Given the short duration of this peak, reliable breeding records are critical, since false-negative results may be obtained if the test is performed too early or too late. A second peak occurs during the last trimester of gestation, with maximum concentrations of 42 ng/mL in the last 30 days before parturition.8,9 The source of estrone sulfate at this stage of gestation is thought to be the fetomaternal unit. Estrone sulfate concentration decreases abruptly with the expulsion of the fetus and its membranes to reach basal values after parturition.9 Estrone sulfate concentration not only confirms pregnancy but may also provide information about the well-being of the fetus.
Relaxin
Blood relaxin concentrations are undetectable until 85 days of gestation but remain above basal thereafter. A first peak (>20 ng/mL) is detected at 3.5 months of gestation. Relaxin concentrations then decrease between 5.5 and 7.5 months, followed by a steady increase until parturition.8 Relaxin is a pregnancy-specific hormone. Its source in South American camelids (SACs) is unknown, but large luteal cells of the mature CL and uterine luminal epithelial cells were identified as the source of relaxin in pregnant dromedary camels.15 Pregnancy diagnosis based on blood relaxin concentration seems possible and accurate 85 days after mating.8 However, no commercial tests are available, and measurement of relaxin remains experimental.
Palpation per Rectum
Palpation per rectum has been described for diagnosis of pregnancy in camelids. However, pelvic anatomy, fat deposition in the pelvic inlet, or size may limit the clinician’s ability to perform palpation per rectum in llamas and alpacas.10 Because of these limitations, palpation per rectum is possible in 90% to 100% of llamas and 70% to 82% of alpacas.10,16 The procedure presents risks that include colonic injuries, rectal irritation, lacerations, or prolapse in the female. Adequate physical or chemical restraint and proper technique are important to prevent these potentially life-threatening complications. Fecal pellets should be removed gently, and 60 mL of lubricant should be instilled into the rectum with a catheter tip syringe. The rectum should be dilated slowly. Local instillation of lidocaine (10 mL injectable 2% xylocaine mixed with 100 mL lubricant) may help decrease reflex straining. Excessive anal tone may be diminished with epidural anesthesia (1 to 2 mL of 2% lidocaine).
Characteristic changes in the size and turgidity of the uterus and in the position of the genital tract within the pelvis and abdomen during gestation occur as a result of increase in the size of the fetus and the volume of fetal fluids. Pregnancy evaluation by palpation per rectum is possible 35 days after mating in llamas, with greater accuracy 45 to 50 days after mating. In alpacas, diagnosis of pregnancy by palpation per rectum may not be possible 30 days after mating, but asymmetry, tension, and turgidity of the uterine horns are palpable at 60 days of gestation.17 At this stage, the left uterine horn doubles in size compared with the right uterine horn. As gestation advances and the size of the gravid uterus increases, it is displaced cranially and ventrally into the abdominal cavity at 120 days. As the uterus becomes more abdominal, the cervix is pulled cranially and can be palpated at the pelvic brim at 180 days. It may be difficult to differentiate between fetal fluids and pathologic uterine contents (pyometra or mucometra) during the early stages of gestation. Palpation of the fetus is possible 90 days after mating, and constitutes the only positive sign of pregnancy in camelids. Fetal head and legs can be identified at 150 days. The fetus lies entirely within the abdomen after 180 days of gestation, and it may be difficult to palpate per rectum at this stage. However, the fetus can be palpated by abdominal ballottement 7 to 9 months after mating. It may be possible to palpate the fetal legs and head within the pelvis 300 days after mating.17 However, in some females, the fetus does not become palpable within the pelvic cavity until a few days before parturition. Since the camelid placenta is diffuse, no placentomes or membrane slip can be palpated.
The accuracy of diagnosis of pregnancy by palpation per rectum is 87% 46 to 110 days after mating and 100% after 150 to 165 days.10,11,18 Palpation per rectum is economical and does not require special equipment. It allows assessment of fetal viability but not well-being. The risks to the female should be considered, and because of size limitations, it is not possible to palpate all females. Accurate evaluation of pregnancy by palpation per rectum is possible, but doing it too late in gestation does not allow efficient reproductive management. Estimation of gestational age or prediction of the approximate parturition date based on findings of palpation per rectum is not reliable.18
Ultrasonography
Real time B-mode ultrasonography is the method of choice for evaluation of pregnancy. It provides the earliest accurate diagnosis; allows for assessment of embryonic and fetal number, development, and well-being; and helps determine the approximate gestational age when the mating date is unknown. Before 60 days after mating, ultrasonography is best performed per rectum. Ultrasonography per rectum is more difficult 87 days after mating, when the gravid uterus has moved too deep into the abdomen.19 A 5- or 7.5-millihertz (mHz) linear transducer is coupled with a rigid extension to allow external manipulation of the transducer in animals too small to accommodate the clinician’s hand.
The embryonic vesicle may be first visualized as early as 9 days after mating as a 2- to 4-mm collection of fluid.20 However, false-negative results may occur early in gestation if the embryonic vesicle is too small to be seen. Evaluation of pregnancy by ultrasonography per rectum is best performed 16 to 23 days after mating, when the accuracy of diagnosis is 100%.14,20 At this stage, the embryonic vesicle appears as a star-shaped anechoic structure on cross-sectional view (Figure 22-1, A). On longitudinal section, it appears as an elongated anechoic structure (see Figure 22-1, B). False-positive results may occur in females with uterine pathologies that result in accumulation of free intrauterine fluid. The number of false-positive results can be minimized if the presence of a CL and nonreceptive behavior are included in the diagnostic criteria. The CL is visualized 3 days after mating as a round structure protruding from the ovarian surface. The CL continues to grow and reaches a larger diameter in pregnant females than in non-pregnant females. It reaches a maximum mean diameter of 14 millimeters (mm; range 12 to 16 mm) 16 days after mating in llamas.21 The CL is less echogenic than the ovarian stroma and may be homogeneous (see Figure 22-1, C) or have a hyperechoic or anechoic central area.
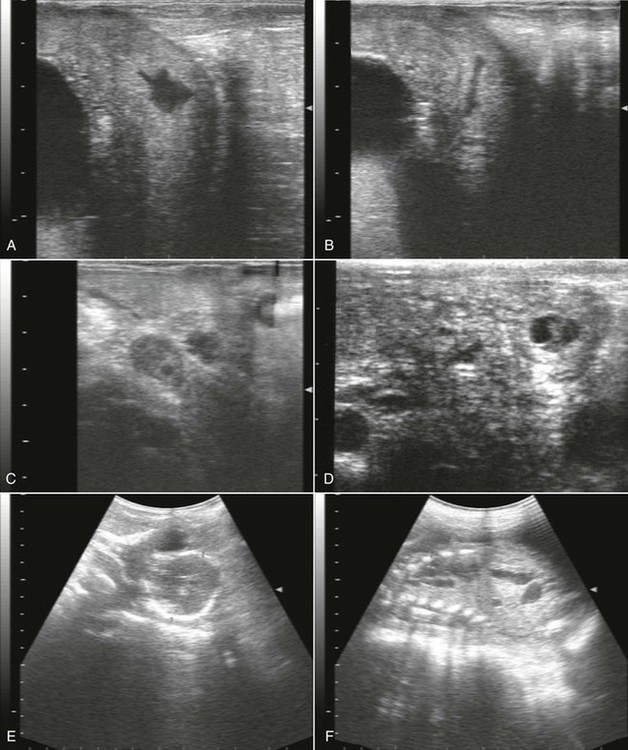
Figure 22-1 Ultrasonographic Images of the Alpaca Conceptus at Various Stages of Gestation.
A, Cross-section view of a 17-day embryonic vesicle (EV). UB, urinary bladder. B, Longitudinal-section view of a 17-day embryonic vesicle. C, Homogeneous corpus luteum (CL). D, A 23-day old embryo proper (arrowhead) and allantoic membrane (arrow). E, Measurement of biparietal diameter in a 292-day alpaca fetus. E, fetal eye. F, Measurement of thoracic width in a 292-day alpaca fetus. H, fetal heart.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


