10 Dermatophytosis in a guinea-pig
CLINICAL EXAMINATION
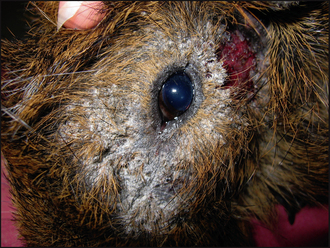
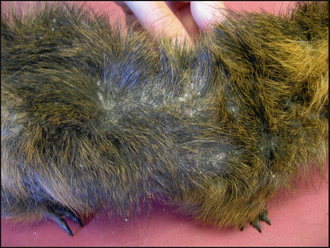
Dermatological examination showed marked crusting and scaling around the left eye (Fig. 10.1), with evidence of inflammation of the skin below the left ear. There was also extension of patchy alopecia and scaling onto the dorsum (Fig. 10.2).
The clinical signs associated with dermatophytosis in guinea-pigs vary. Lesions often begin with circumscribed or coalescing oval to patchy areas of non-pruritic scaling and alopecia, usually affecting the nose (Fig. 10.3), ears and face. However, in advanced cases lesions may spread to involve the neck, limbs and body. If untreated, then the lesions can become inflamed and infected with bacteria. This secondary infection will manifest as pustules, papules and crusts, usually with an increase in the pruritus, although the level of irritation does differ between individuals. Dermatophytosis can occur concurrently with Trixacaris caviae infestation; in these cases, the pruritus tends to be severe.
CASE WORK-UP
Skin/hair scrapes: Multiple skin scrapes were taken from around the left eye and the dorsum. These were mounted in liquid paraffin on a microscope slide with a coverslip and examined under ×40 magnification. No ectoparasites were seen. This absence does not entirely rule out ectoparasites and, in general, it may be useful to repeat the tape strips, or even consider prophylactic antiparasite treatment.
Further investigations aimed towards diagnosing dermatophytosis:
Sterile toothbrush: Samples may be obtained for microscopy or culture by grooming the hairs on and around the lesions with a sterile or new toothbrush.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree