16 Cytology of Inflammatory or Neoplastic Masses
Role of Cytology in Diagnosis
Of 147 skin tumors, only 105 (71%) cytologic diagnoses agreed with histologic diagnoses.7 Exceptions exist in which cytology is as diagnostic as (or more diagnostic than) histology (e.g., individual cell detail of leukemias is more diagnostic than tissue patterns). Cytology was correct in 60 of 64 round cell tumors and occasionally more diagnostic than histology.6 Cytologic and histologic diagnoses agreed on all of the following tumors: 37 mast cell tumors, 11 melanomas, 2 histiocytomas, and 1 cutaneous lymphoma.7 Accurate cytologic diagnoses were also made for squamous cell carcinomas, lipomas, and metastasis to lymph nodes.
Cytologic Techniques
Sample Collection and Slide Preparation
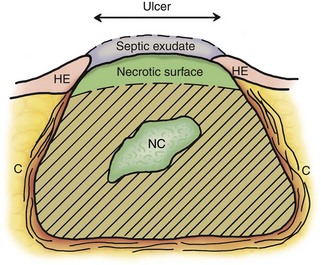
Proper sample collection and slide preparation are absolutely necessary and are a common limiting factor in cytologic diagnosis. Cytologic diagnosis requires an adequate number of cells with good morphology that represent the mass. A fine-needle aspirate (FNA) should be obtained from an area of the mass that reflects the primary problem. The site being sampled determines what material is collected (Figure 16-1). An impression smear from the surface over a mass often has only exudate, bacteria, and necrotic or reactive cells that do not reflect the primary mass. Similarly, a wash over the mass (e.g., nasal flush, bronchoalveolar lavage [BAL]) often collects only exudates and reactive cells on the surface. Pus (septic exudate) may be misleading and suggest the mass is an abscess. FNA samples of deeper tissue are more likely to be diagnostic.
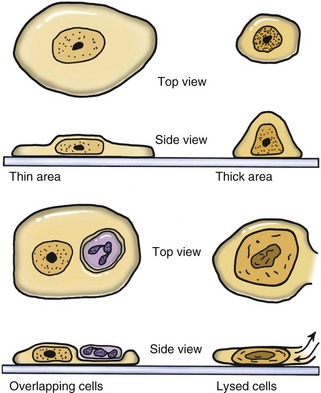
Cells in a thin area (monolayer of cells) spread out flatly and expose a large surface area to view (Figure 16-2). If the smear is thick, cells are supported more upright on the slide and have a smaller diameter. Cells in thick areas are taller (thicker) and thus stain darker, often too dark to see any detail. Protein-rich fluid, necrotic debris, or ultrasound gel surrounding cells interferes with staining. If fluid is viscous, a squash preparation may help to get a thin smear. A drop of fluid is placed between two slides; while the drop spreads to its maximum diameter, the slides are slid apart while the two surfaces are in contact with each other. This creates a smear on each slide. Lymphoid cells and cells from necrotic centers are very fragile and easily lysed beyond recognition. Use of a coverslip for a squash preparation or blood smear–type smear often causes less damage to cells.
A nonaspirate (capillary) technique is easier to perform and yields less bloody samples of equal or greater cellularity, especially from highly vascular tissues (e.g., liver aspirates).9 The technique described here is a modification of the nonaspiration technique used by Dr. Rick Cowell while at Oklahoma State University. Air (i.e., 5 ml) is aspirated into a 10-ml syringe, and a 22-gauge needle is attached. The syringe is held at the base of the needle to allow for better control, and the mass is stabilized with the operator’s free hand. The needle is introduced into the mass and rapidly moved back and forth along the same tract five or six times. Negative pressure (i.e., aspiration) is not applied. The cells are collected by shearing and capillary action. The needle is withdrawn from the mass, and collected cells are quickly but gently expelled onto a clean glass slide by depressing the plunger. The collected material is then spread out as discussed earlier. A common error is the “shotgun spray” in which the small volume of fluid in the needle is sprayed on to the glass as small droplets (like the pattern of shotgun shot hitting a target). These small thick drops dry too quickly to be drawn out into thin smears. Instead, the operator should touch the end of the needle to the glass and gently express the contents out as one drop that is then quickly and gently spread it out as a thin smear. Generally, only one smear can be made from each collection attempt. Therefore three or four collections from different sites should be taken. An alternative is a “packing” technique where only a needle (without syringe attached) is passed through a mass to pack the needle with cells with no aspiration.
Stains
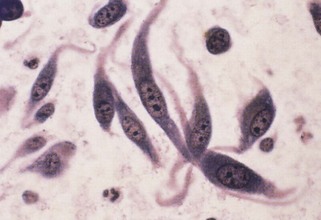
NMB is a monochrome stain with variably intense blue staining. A “wet mount” is made by placing a drop of NMB on an air-dried smear and applying a coverslip. To prevent retention of an air bubble over part of the smear (usually the most diagnostic area), the coverslip should be used to gently pull the drop of NMB over the cells to moisten them before slowly applying the coverslip. Staining is immediate. NMB stains nuclear material well and demonstrates distinct chromatin patterns (Figure 16-3). This nuclear detail is very useful in evaluating malignant criteria. The transparent nature of NMB is a major advantage, because one can see through thicker tissue fragments that would be too darkly stained with a Wright stain. The microscope can be focused through different depths of tissue fragments to judge individual cell detail and architectural patterns in three dimensions. Tissue fragments are often the most diagnostic material on smears from neoplasms but stain too darkly to evaluate with a Wright stain. The fragments are like tiny biopsy sections that allow evaluation of how cells were oriented in the mass. These architectural patterns help identify the tissue type. One can evaluate adjacent cells for true variability suggesting malignancy, compared with the variability of isolated cells on a smear that may have come from different areas or cell types in the mass.
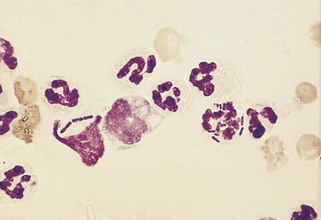
In summary, NMB is excellent for nuclear detail, thick tissue fragments, and most fungi. Wright stain is excellent for inflammatory lesions, because the stained appearance of leukocytes (white blood cells [WBCs]) is similar to that in blood smears and bacteria consistently have a characteristic dull blue color (Figure 16-4). Although a Wright stain is not as good as NMB for nuclear detail, it is acceptable for evaluating tissue cells for criteria of malignancy and excellent for bacteria and most fungi (Figure 16-5). Use of both Wright and NMB stains for the same lesion works well, because different characteristics of the cells are illustrated by different stains.
Other stains may be used. Sudan stain is useful for diagnosis of fatty liver, chylothorax (Figure 16-6), aspiration pneumonia (Figure 16-7), or lipid granulomas. A drop of Sudan stain (or other neutral fat stain) is drawn over a smear to stain the cells and background selectively for lipid. Excess stain is poured off and then the smear is counter-stained with a drop of NMB to show adequate cell detail. NMB stains the nucleus and other cell structures blue, and the Sudan stains neutral fat red.
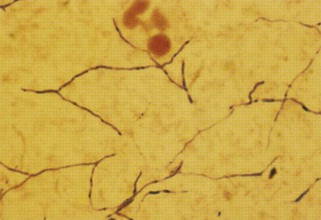
Veterinarians sometimes request that their cytologic samples are Gram stained because different antibiotics are used for gram-positive versus gram-negative bacteria. However, Gram staining is absolutely not recommended for cytologic smears. Gram staining is inconsistent for bacteria in exudate. In thick smears the bacteria may not decolorize, creating a false impression that the bacteria are gram positive. In thin smears, bacteria may decolorize too much, suggesting they are gram negative. Gram staining is not sensitive for screening cytologic smears for gram-negative (i.e., red) bacteria in low numbers in a red, proteinaceous background (Figure 16-8). Wright-Giemsa–type stains consistently stain bacteria blue and are greatly preferred for finding bacteria on cytologic smears. Wright-Giemsa stains allow easier detection and definition of size and shape. In general, coccoid bacteria are likely gram-positive Staphylococcus and Streptococcus. Gram staining is best restricted to bacteriology laboratories where smears are consistent in thickness and staining is interpreted daily on bacteria where the final classification is determined daily. Gram stain differentiates gram-negative from gram-positive bacteria well on uniformly thin smears from cultures on blood agar plates. Acid-fast stain is rarely needed, because mycobacterial infections are uncommon (see Figure 16-8). Mycobacteria are unique in that they do not stain at all with Wright-Giemsa stains because of their waxy coat (Figure 16-9).
Cytologic Conclusions
The usual composition of a mass is a proliferation of tissue cells, an accumulation of inflammatory cells, or both. Miscellaneous masses include hematomas, cysts, or focal areas of necrosis. A general approach to cytologic interpretation of a case is simplified in Figure 16-10. More complete description of many cytologic diagnoses is available.11
Slide Reading Approach
Most American veterinarians have ample microscopy, histology, and pathology training, so most can learn to make many cytologic diagnoses as long as they recognize their limitations and continue to learn from their cases. Cytologic evaluation may be performed on excised masses and then the descriptions and conclusions may be compared with histopathology reports. Cytologic evaluation is a visual task, so one should obtain one or more cytologic atlases for frequent reference in the lab.2,5,11 An organized approach to an aspirate or impression smear of an abnormal mass is necessary for consistent conclusions. A summary of steps follows, and details are provided in later discussions.
1 Establish that a sufficient number of intact, properly stained cells are present and properly represent the mass.
2 Scan smears at low power to determine variation in distribution and content. Look for large structures (e.g., fungus, bacterial colony, parasite eggs, or larvae).
3 Begin fine evaluation of cells in a thin area with intact cells of good staining quality.
4 Determine whether the cell population is primarily inflammatory. If so, attempt to identify the etiologic agent.
5 Determine whether enough tissue cells of one type are present to indicate a noninflammatory, proliferative tissue mass.
6 With proliferative tissue masses (e.g., neoplasia or hyperplasia), determine cell types (connective tissue, epithelial, round cell) present and amount of evidence indicating malignancy.
Inflammatory Masses
Neutrophilic Inflammation
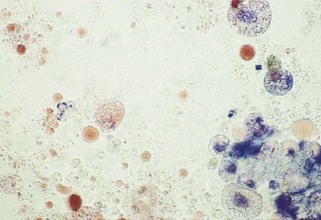
Neutrophilic infiltrates (e.g., exudation, suppuration, abscess formation, purulent inflammation) are so frequently seen that they are almost synonymous with inflammation. Neutrophils are the most motile WBCs and the first to infiltrate an area or exude out on a surface. Some call neutrophilic inflammation “acute inflammation,” even though neutrophils may be prominent in chronic but active inflammation. Therefore the term acute may refer to a cell type (e.g., predominance of neutrophils) and not always to a time interval. Pus is proteinaceous fluid with many neutrophils and cell debris. Aspiration readily collects this fluid material; therefore many neutrophils are often present on smears. Other cells in an inflammatory mass may not exfoliate as easily (especially fibroblasts) if scarring and fibrosis are present. Neutrophils are associated with bacterial infections and some yeast infections (e.g., Candida), but nonseptic causes include immune-mediated processes (e.g., lupus polyarthritis) and chemical irritation (e.g., pancreatitis, bile peritonitis). A neutrophil migrating between stratified squamous epithelial cells may indent into the surface of a squamous cell and appear as if it is within the squamous cell when it exfoliates (see Figure 16-2).
Bacterial Sepsis
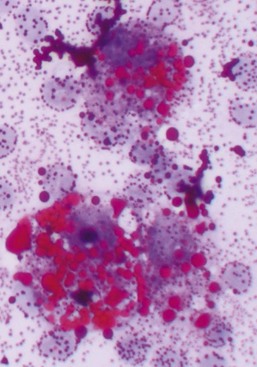
Neutrophilic inflammation indicates a search for bacteria. The best place to search is in cytoplasm of neutrophils (see Figure 16-4). The neutrophil’s cytoplasm is usually clear and free of granular debris. Macrophages often contain phagocytized cell debris that can mimic bacteria. Bacteria are more prominent in the clear neutrophilic cytoplasm, and the phagocytic vacuole may help outline the organism. Bacteria have uniform shapes and sizes, in contrast with granular debris. Formation of uniform pairs, tetrads, and chains identifies structures as bacteria. Rods are more confidently identified as bacteria than cocci. Wright stain precipitate is coccoid in appearance and may mimic coccoid bacteria. However, the irregular size of the precipitate, a more purple color, and a refractile appearance will differentiate stain precipitate from bacteria. Bacteria have a more dull blue color (see Figure 16-4). Stain precipitate may be on neutrophils, suggesting phagocytosis, but will also be elsewhere on the glass slide including where no sample was applied.
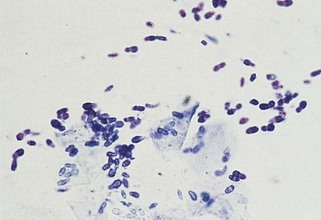
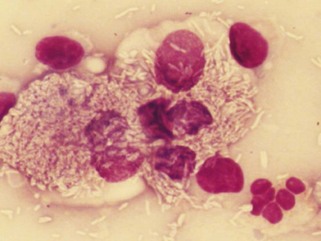
The description of bacteria should include number, location (e.g., free in the smear, phagocytized by neutrophils, or on epithelial cells), appearance, and whether a pure or mixed population is present. These observations permit certain conclusions. For example, a pure population of small cocci in chains within neutrophils from an abscessed lymph node suggests an infection (e.g., Streptococcus), whereas a mixed population of variably sized, large rods and even cocci in neutrophils in abdominal fluid suggests a ruptured gut. Beaded filamentous organisms indicate higher bacteria (e.g., Actinomyces) (Figure 16-11; see also Figure 16-8).
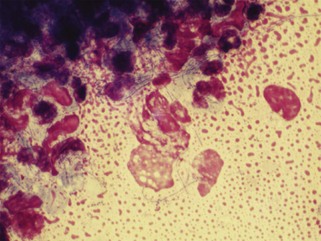
Bacteria on stratified squamous epithelial cells are usually normal flora from a body surface. A normal flora of the oropharyngeal area of dogs is Simonsiella. Finding this characteristic, huge, flat multicellular form (called trichrome) of 12 to 20 bacterial cells indicates at least part of the sample came from the mouth or pharynx. Finding Simonsiella and a mixture of other bacteria on squames indicates that one cannot trust finding bacteria in another area of a transtracheal wash or BAL as indication of infection of the lower respiratory tract. Bordatella bronchiseptica has a predilection for cilia of respiratory epithelial cells, so finding small rods on cilia suggests Bordatella infection (Figure 16-12).
Degenerative Neutrophils
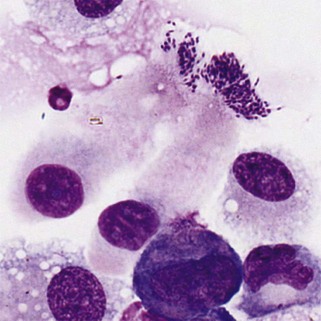
How long should one search for bacteria in a sample with neutrophilic inflammation? Bacteria may be in low concentrations in chronic infections, with antibiotic therapy, and in some samples such as joint fluid and cerebrospinal fluid (CSF). Five to 10 minutes is a reasonable time limit unless something suggests searching longer. For example, one should search for bacteria longer than usual if neutrophils appear degenerate. Bacterial toxins often cause rapid neutrophil death (karyolysis). Degenerative changes in neutrophils suggest but do not prove sepsis. Some bacteria seem less toxic to neutrophils, however, and bacteria may be found in nondegenerate neutrophils (see Figure 16-4).
Morphologically degenerate neutrophils are characterized by swelling of the nucleus (karyolysis) and cytoplasm. Karyolysis appears as a wider, more irregularly shaped, lighter-staining nucleus lacking the dark, distinctly granular chromatin pattern and thin lobulated shape of viable nuclei (see Figure 16-11). Severely degenerate neutrophils may barely resemble neutrophils as they swell and lyse into “globs” of nuclear debris. Degenerative changes caused by bacteria must be differentiated from swelling due to sample storage, trauma to fragile cells during streaking of the smear, or nonbacterial toxic effects (e.g., urine). Inexperienced cytologists tend to over-identify degenerative neutrophil changes by examining damaged cells (see Figure 16-4). One should evaluate only intact, undamaged cells. If the neutrophils with intact cell boundaries appear nondegenerate, lysed neutrophils on the slide are probably artifactually broken rather than degenerate from bacterial toxins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree