Chapter 86 Cyanide
INTRODUCTION
Another possible source of cyanide exposure is enclosed space fires. Combustion of many substances such as nylon, plastics, wool, and silk may release hydrogen cyanide gas.1 Therefore victims of smoke inhalation are at risk for cyanide poisoning in addition to carbon monoxide poisoning2 (see Chapters 28 and 87, Smoke Inhalation and Carbon Monoxide, respectively).
MECHANISMS OF TOXICITY
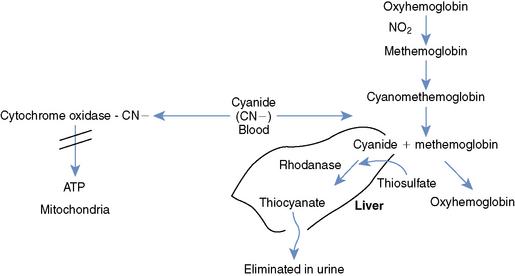
Cyanides are present in low concentrations in the environment; therefore several intrinsic biochemical pathways for cyanide detoxification exist. The most important route for cyanide excretion is through the formation of thiocyanate, which is subsequently excreted in the urine through the kidneys.1 Thiocyanate is formed primarily in the liver, directly through the activity of the rhodanase enzyme and indirectly via the enzymes 3-mercaptopyruvate sulfurtransferase and thiosulfate reductase.1,3 These enzymes are responsible for combining cyanide and sulfur to form thiocyanate. Despite these intrinsic mechanisms for cyanide detoxification, these enzyme systems are easily overwhelmed during cyanide poisonings because of the body’s limited supply of sulfur (Figure 86-1).1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree