(1)
Arezzo, Italy
In collaboration with Walter Bertazzolo (dipl. ECVCP)
5.1 Introduction
In this chapter, the cutaneous metastases of non-primary skin neoplasms, namely those originating from tumors of the internal organs, are discussed. Skin metastases of internal malignancies are, with some exceptions, very rare and only sporadic cases of metastases from gastric, intestinal, prostatic and bronchial carcinomas, from testicular seminoma and from some sarcomas such as haemangiosarcomas and osteosarcomas, have been reported in the veterinary literature. The spread of neoplastic cells occurs mainly via the bloodstream, and therefore, metastases can develop on any part of the skin. The most frequently observed cutaneous metastases are those linked to the bronchogenic carcinoma in cats, which characterise the so-called lung–digit syndrome. In this neoplastic disease, metastases are multicentric and mostly localised to the digits and muscles. Although mammary tissue is cutaneous adnexa, because of the importance and the enormity of the topic, mammary cancers are generally dealt with in textbooks exclusively dedicated to this subject. In dogs, as in human beings, a clinical variant of mammary neoplasia called inflammatory mammary carcinoma is well documented. This neoplasia, shows clinical signs very similar to other primary skin cancers or to nodular non-neoplastic skin diseases, and it is characterised by the lymphatic spread of neoplastic cells. For this reason, this variant of mammary carcinoma is discussed in this chapter. The metastasis of round cell tumors, such as mast cell tumours, lymphomas, histiocytic diseases and multiple myeloma, are not included in this chapter. In these haematopoietic tumours, the simultaneous presence of lesions in the internal organs and on the skin is considered a common occurrence. Furthermore, it is not always possible to define when the skin neoplasia is primary or metastatic. The neoplasms included in this group of tumors have been extensively covered in Chap. 4.
5.2 Metastatic Pulmonary Carcinoma in Cats (Lung–Digit Syndrome)
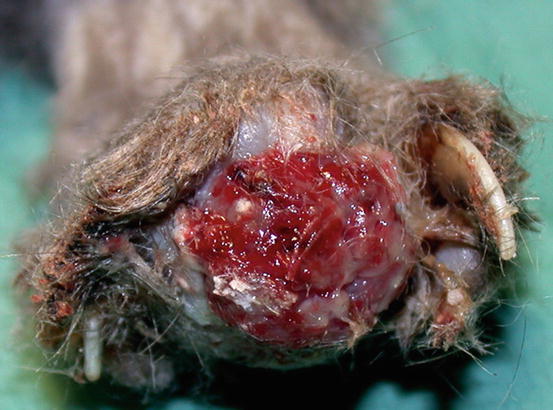
The term lung–digit syndrome (LDS) is used to describe a clinical syndrome characterised by skin metastases secondary to the diffusion, via the bloodstream, of a primary bronchogenic carcinoma (Estrada and Lagadic 1992; Gottfried et al. 2000; van der Linde-Sipman and van den Ingh 2000). Adult to old cats are more predisposed, although, in rare cases, LDS has been reported in very young patients. In most cases LDS is characterised by the development of multiple lesions, mainly located on several digits of different feet; the digits appear swollen, ulcerated, discharging purulent exudate mixed with blood, painful on palpation and with deviation of the axis of the nail and/or onychomadesis (Figs. 5.1 and 5.2). The neoplastic cells cause osteolysis and cats develop lameness.
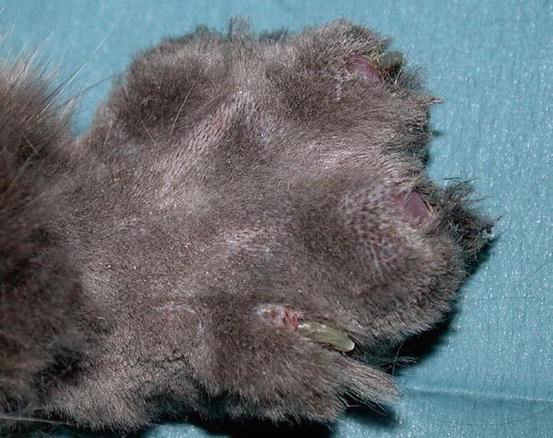

Fig. 5.1
Multiple digital swelling in a Persian cat with lung–digit syndrome (LDS)
In some cats, extra-digital cutaneous localisation, both as a single lesion and in combination with the digital lesions, are reported (Favrot and Degorce-Rubiales 2005; Petterino et al. 2005; van der Linde-Sipman and van den Ingh 2000) (Fig. 5.3). Some cases of extra-skin metastases, predominantly affecting skeletal muscles and bones, have also been reported (Goldfinch and Argyle 2012; Langlais et al. 2006). Thoracic radiographs usually show a primary lung cancer, mostly single and well circumscribed; in rare cases, it is possible to find multiple lesions, whereas in other patients, the cancer is not radiographically detectable, especially when the diaphragmatic lobe of the lung is affected (Fig. 5.4) (Barr et al. 1987). Most cats do not show respiratory symptoms (Moore and Middleton 1982). The angioinvasive power of this type of cancer, and the fact that cats have a high digital blood flow that permits better heat dissipation, are assumed to be the cause of the multiple digital metastases (Moore and Middleton 1982).
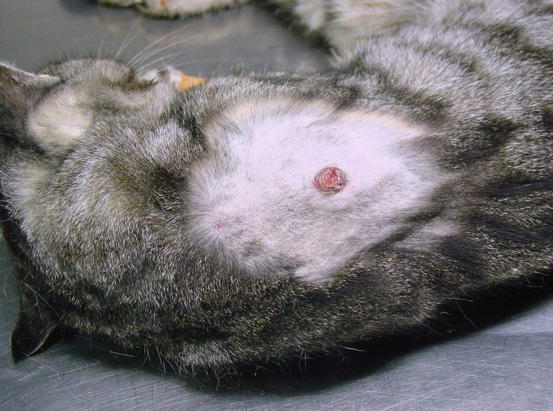
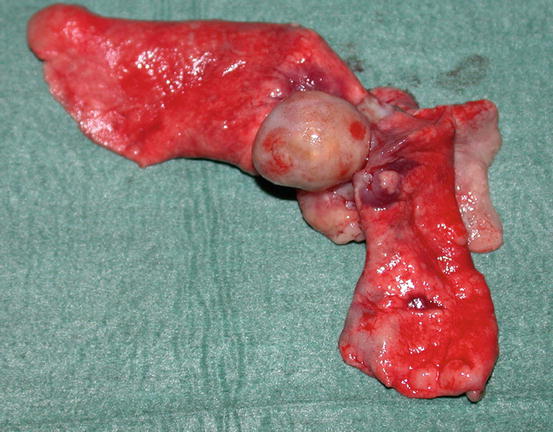

Fig. 5.3
Case of LDS: small and ulcerated cutaneous nodule on the chest of a cat. The primary neoplasm was confirmed as a bronchogenic carcinoma

Fig. 5.4
Primary bronchial carcinoma. The small neoplasia was not evident to the radiographic investigation
Cytological Findings
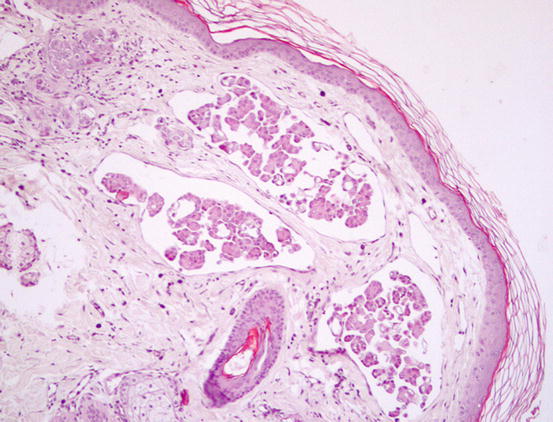
Three histological subtypes of bronchial carcinoma are recognised as causes of lung–digit syndrome: adenosquamous (mucoepidermoid) carcinoma, squamous cell carcinoma and adenocarcinoma. The first two seem to be the most frequent subtypes (Fig. 5.5) (Gross et al. 2005).
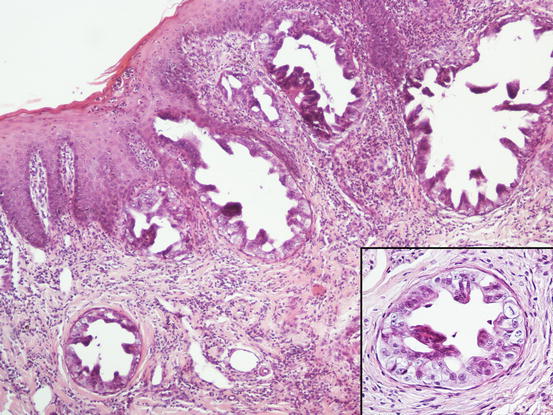

Fig. 5.5
Histopathology of the skin metastasis of a bronchogenic carcinoma: multiple cystic glandular formations composed of polygonal to columnar epithelial malignant cells. Neoplastic intravascular thrombus (inset)
The cytology specimens are characterised by clusters of epithelial cells, more or less cohesive, from polygonal to columnar in shape, which show varying features of atypia such as anisocytosis, anisokaryosis, pleomorphic nuclei and prominent nucleoli. The presence of acinar or tubular arrangements allow identification of the glandular nature of the neoplasia, but the diagnosis of metastatic bronchial carcinoma can only be made when the ciliated and/or goblet cells (the mucin-secreting cells) are observed (Figs. 5.6, 5.7, and 5.8). The latter are recognisable by the presence of vacuoles or of intracytoplasmic amorphous and coarse basophilic material, representing the mucous secretion (Fig. 5.9). These two cell types are more common in the adenocarcinomatous subtype, and, to a lesser extent, in the adenosquamous carcinoma variant. As the adenocarcinoma is the less frequent subtype of LDS, the detection of ciliated cells is not a constant finding; therefore, when they are not present on slides, the cytological diagnosis of metastatic bronchial carcinoma is not possible. In specimens collected from the adenosquamous subtype, it is possible to detect some epithelial cells showing squamous differentiation; the latter are the predominant cytological population in the histological SCC subtype (Figs. 5.10 and 5.11). For these reasons, cytological differentiation between a primary cutaneous SCC of the nail bed and digital metastasis from a bronchogenic SCC is very difficult and may be impossible. However, the detection of clusters with a glandular arrangement and the clinical aspect of multiple digital lesions lead to suspected LDS.
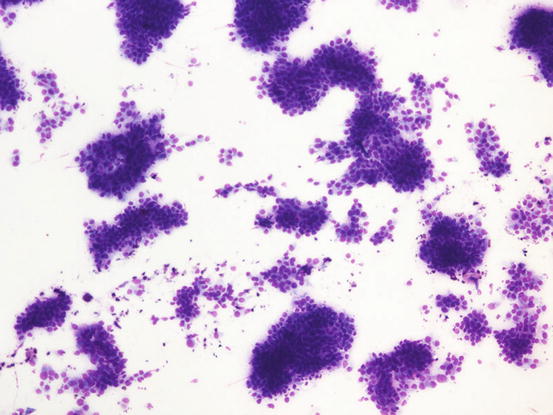
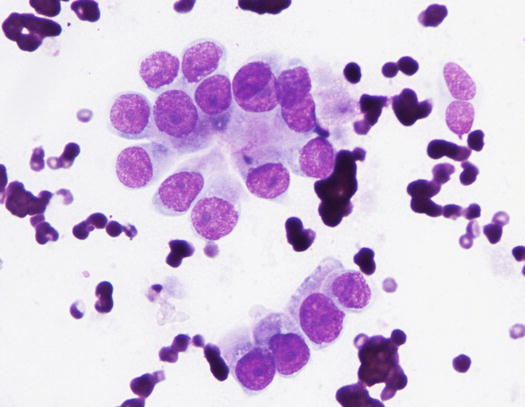
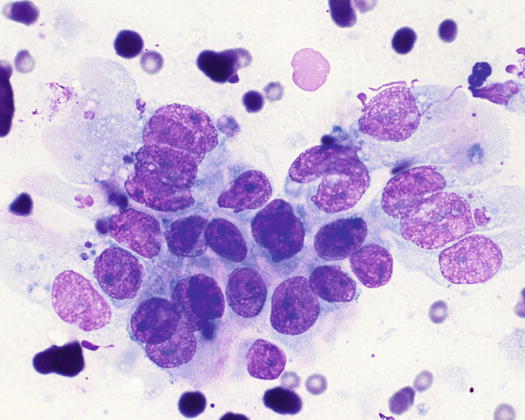
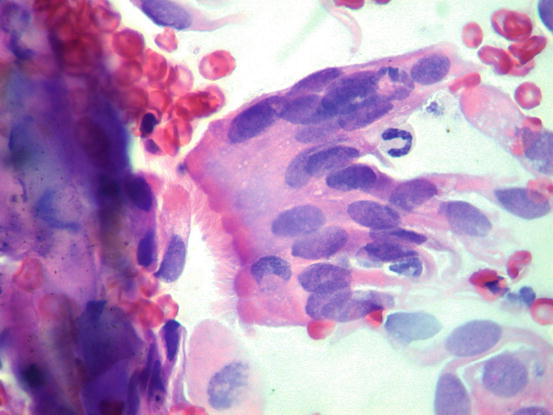
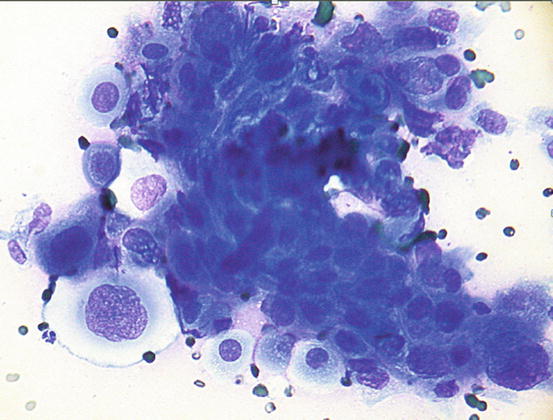
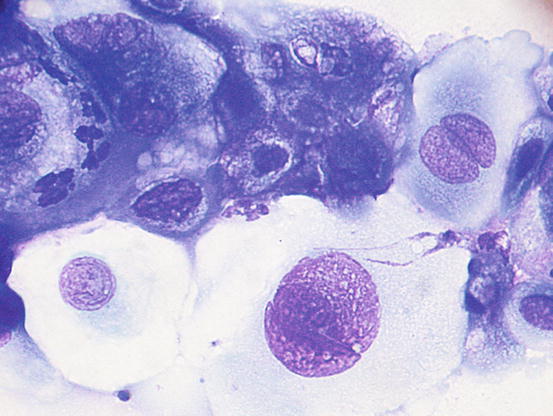

Fig. 5.6
Cytology of LDS: multiple cohesive clusters of epithelial carcinomatous cells

Fig. 5.7
Cytology of LDS: clusters of cuboidal bronchial cells showing acinar and palisading arrangements

Fig. 5.8
Cytology of LDS: clusters of columnar bronchial cells showing acinar and palisading arrangements. The goblet cells show intracytoplasmic amorphous and coarse basophilic material

Fig. 5.9
Cytology of LDS: clusters of columnar bronchial cells. Cilia are clearly evident on the apical part of the cells (Courtesy of Dr. F. Carrani, Italy)

Fig. 5.10
Cytology of LDS: clusters of malignant bronchial cells showing squamous differentiation

Fig. 5.11
Cytology of LDS: at high magnifications, squamous cells are more evident
5.3 Cutaneous Metastasis from Inflammatory Mammary Carcinoma
Inflammatory mammary carcinoma (IMC) is the most malignant, aggressive and lethal variant of mammary cancer in dogs and it is characterised by a clinical presentation that is completely different from that of other mammary carcinomas (Marconato et al. 2009; Perez Alenza et al. 2001). In human beings, studies on inflammatory breast cancer showed the high angiogenic and angioinvasive potential of the tumour, which contributes to its aggressive power (Kleer et al. 2000).
Although mammary carcinomas in dogs frequently metastasise to internal organs, the IMC is characterised by its ability to spread neoplastic cells through the cutaneous lymphatic vessels.
Grossly, the cutaneous metastatic lesions may appear as diffuse erythematous swelling of the skin, erosions and multiple, poorly defined and often confluent nodular–papular or nodular lesions (Figs. 5.12, 5.13, 5.14, and 5.15). The metastasis centrifugally expanded from a primary mammary neoplasm, but primary neoplasia is not always clinically evident and, in these cases, the IMC is suspected when the above-mentioned lesions are observed in the mammary area (Clemente et al. 2010). As the neoplasia rapidly metastasises via the lymphatics, in many dogs, the lesions can be observed far from the mammary area, such as the inner surface of the thighs and axilla and on the lateral area of the chest and shoulders. It must be pointed out that the clinical aspects of the skin lesions explain why the adjective inflammatory has been coined to define this variant of mammary cancer. The adjective is indeed related to the macroscopic characteristics of the cancer and it is absolutely not linked to the microscopic presence of inflammatory cells in the context of the neoplastic proliferation.
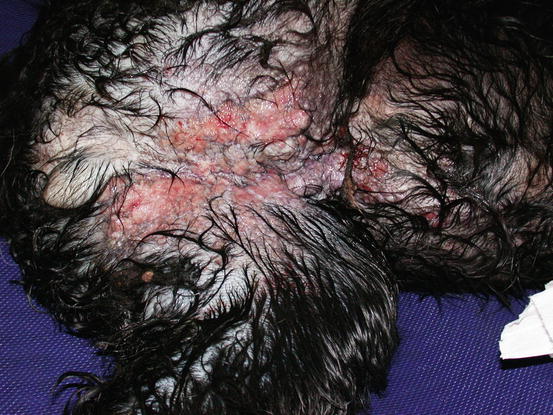
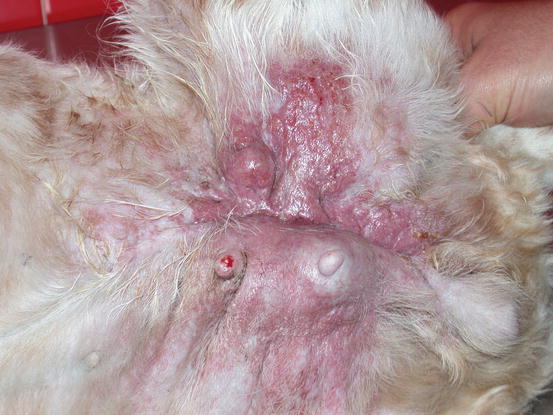
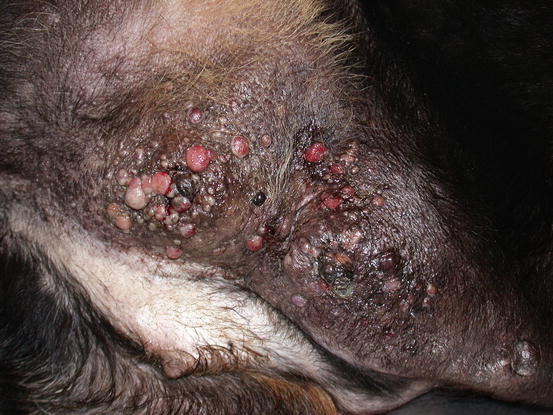
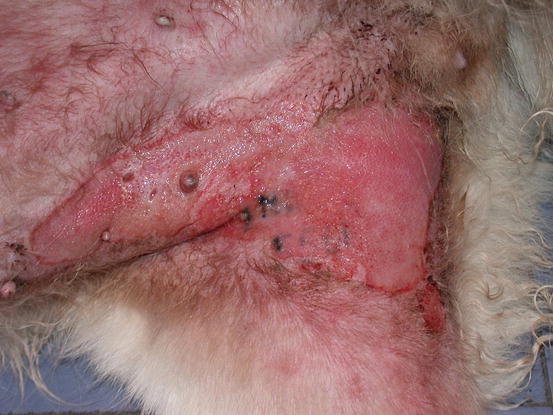

Fig. 5.12
Multiple, small, erythematous and ulcerated nodular papules and plaques, on the abdomen and inner thigh of a Cocker with an inflammatory mammary carcinoma (IMC). The wet hairs are due to excessive licking because of the strong pruritus

Fig. 5.13
Swelling and erythema of the skin covering a large mammary neoplasia. Note that the lesions are spread over the left thigh forming a large plaque

Fig. 5.14
Multiple neoplastic nodular papules and nodules on the abdomen and leg of a Rottweiler affected by an IMC

Fig. 5.15
Large erythematous skin ulceration on the right legs of a mixed-breed dog: unusual presentation of an IMC
Cytological Findings
The microscopic feature of IMC is the spread of neoplastic cells through the lymphatic vessels, which can be easily diagnosed via histopathology (Figs. 5.16 and 5.17). This metastatic behaviour justifies why the neoplasia is also defined as carcinomatous lymphangiectasia. The cytological samples collected from papular–nodular lesions show highly cellular specimens usually characterised by a clean background, sometimes slightly haemocontaminated, with many clusters of malignant epithelial cells showing various features of malignancy, mostly anisocytosis, anisokaryosis, voluminous nuclei, macronucleoli, nuclear moulding, cannibalism and vacuolated cytoplasm of varying sizes that can contain granular secretion (Fig. 5.18). Many clusters of epithelial carcinomatous cells show a loss of cohesiveness; in these cases the intercellular spaces are more evident and sometimes,small clusters composed of a few cells or discrete neoplastic cells are observed (Fig. 5.19). The finding of acinar arrangements of the cells, especially in poorly differentiated carcinomas, helps to interpret the glandular origin of the neoplasia (Fig. 5.20). Single cells containing large secretory intracytoplasmic vacuoles that marginalise the nucleus, the so-called signet ring cells, are also often detected.