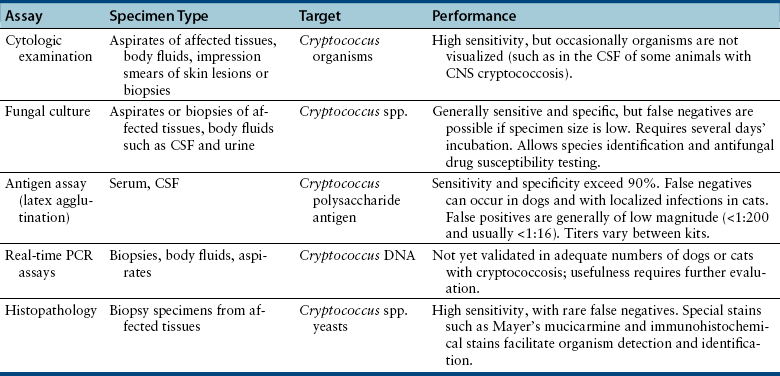
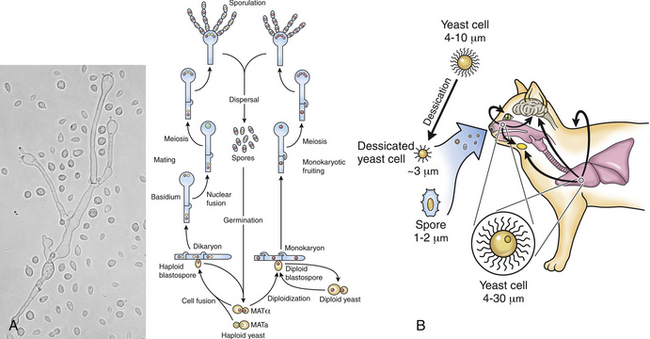
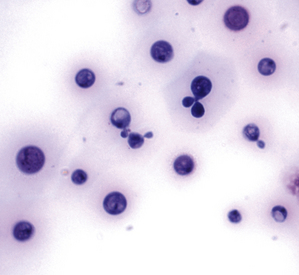
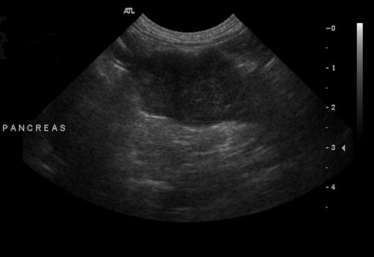
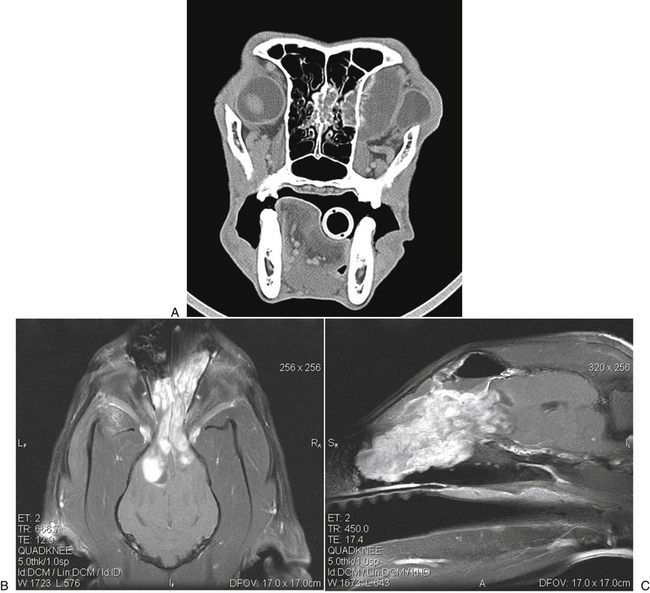
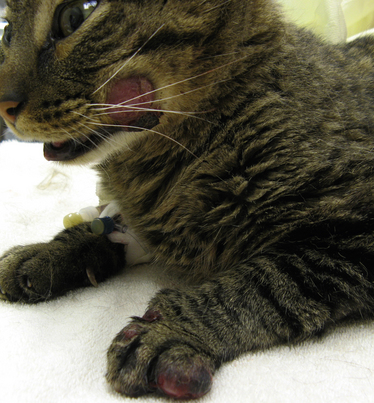
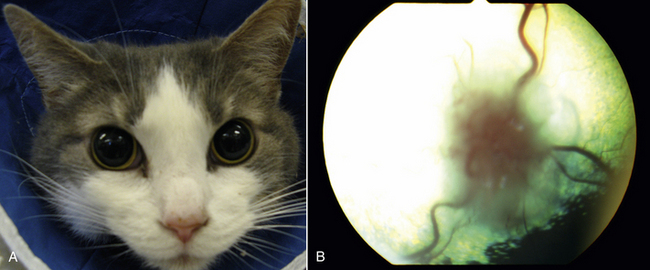
Chapter 62 Cryptococcosis is an important fungal infection of people and animals worldwide and the most common systemic mycosis of cats. The infection is acquired from the environment, and disease transmission from one animal to another has not been reported. Occasional outbreaks of cryptococcosis in people and animals may result from exposure to common environmental sources.2,3 Various domestic and native mammals may be infected, which include cats, dogs, ferrets, horses, camelids, goats, sheep, cattle, dolphins, birds, koalas, and other marsupials.4–10 The incidence of cryptococcosis in cats is eightfold higher than that in dogs.11 The genus Cryptococcus contains at least 19 encapsulated yeast species. Cryptococcus neoformans and Cryptococcus gattii (formerly C. neoformans var. gattii) cause the vast majority of infections in dogs, cats, and humans. C. gattii has emerged as a pathogen of immunocompetent humans in temperate regions of North America, in particular on the west coast of the United States and in British Columbia, Canada. Other species, particularly Cryptococcus laurentii and Cryptococcus albidus, rarely cause disease in people and animals, but may be emerging as a result of immunocompromise.12–14 Cryptococcus magnus was isolated from the external ear canal of a cat with otitis externa,15 and C. laurentii has been isolated from the external ear canal of dogs.16 Cryptococcus spp. are dimorphic, basidiomycetous fungi. The life cycle of the fungus is thought to involve growth in host tissues as an encapsulated haploid budding yeast, combined with the ability to undergo a transition to a filamentous form (or teleomorph) in the environment. Two mating types of Cryptococcus spp. exist, α and a. The α-type is vastly more prevalent in environmental and clinical samples. Under appropriate laboratory conditions, the two mating types fuse and adopt a dikaryotic filamentous state, also known as the perfect state. This is followed by production of basidia, which are small, club-shaped structures on which basidiospores form (Figure 62-1).17 Cells of the α mating type can also undergo asexual reproduction via a process known as monokaryotic fruiting, which also involves filamentation and spore formation.17–19 Although they have never been observed in nature, basidiospores (or possibly desiccated yeasts) are thought to be the main form that, when inhaled, gives rise to mammalian disease.20 In animal tissues, basidiospores convert to the yeast form, which is round to oval with a variably sized capsule. The capsule is primarily (90% to 95%) composed of a polysaccharide known as glucuronoxylomannan (GXM), with smaller amounts of galactoxylomannan. The capsule enlarges after infection of mammalian hosts and protects the fungus from phagocytosis and environmental insults such as desiccation. Within tissues, Cryptococcus reproduces by forming one or two daughter cells (buds) that are connected to the parent cell by a narrow base (Figure 62-2). FIGURE 62-1 Life cycle of Cryptococcus spp. A, Spores result from either mating between cells of opposite mating types (MATa or MATα) and subsequent formation of dikaryotic hyphae or unisexual mating of MATα cells (monokaryotic fruiting). In both cases, sexual development within the filamentous form results in meiosis and basidiospore formation. The spores germinate to produce haploid, yeast-like cells that divide by budding. B, Spores, and potentially desiccated yeast cells, are inhaled. Germination of spores or vegetative growth of yeast cells in the upper or lower respiratory tract results in the proliferation of budding yeasts. Dissemination to a variety of tissues can then occur, but especially to the lymph nodes, nasal cavity, skin, and CNS. (Modified from Kronstad JW, Attarian R, Cadieux B, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol 2011;9:193-203. Basidiospore image courtesy Patrik Inderbitzen, University of California, Davis.) FIGURE 62-2 CSF cytology from an 8-year-old male neutered domestic shorthair cat with cryptococcal meningitis. Large numbers of budding cryptococcal yeasts are present. Historically, five serotypes of Cryptococcus (A, B, C, D, and AD) were recognized on the basis of antigenic differences in the capsular polysaccharide. Based on the results of molecular typing methods such as PCR fingerprinting and multilocus sequence typing, cryptococcal strains from around the world are divided into eight major molecular types: VNI and VNII (C. neoformans serotype A); VNIII (C. neoformans serotype AD); VNIV (C. neoformans serotype D), and VGI, VGII, VGIII, and VGIV (C. gattii, serotypes B and C).21 Hybrid strains also exist. Differences in epidemiology, pathogenicity, clinical features, and drug susceptibility have been associated with each species and molecular type. Cats of all ages may be affected,22 but young adult cats appear to be at increased risk. In dogs, cryptococcosis is diagnosed predominantly in young adult dogs. In one study, the median age of cats and dogs was 6 and 4 years, respectively.11 No clear sex predisposition exists. Siamese, birman, and ragdoll cats have been predisposed in Australian studies but were not found to be at risk in the United States.11,23 Similarly, German shepherd dogs, Doberman pinschers, and great Danes appear to be predisposed in Australia, whereas in the United States, American cocker spaniel dogs are strongly predisposed.5,11,22,24,25 Most cats and dogs with cryptococcosis do not have identifiable underlying immunosuppressive illness. The prevalence of retrovirus infection in cat populations with cryptococcosis does not differ from that in cat populations without cryptococcosis.11,22 However, some cats with cryptococcosis that are co-infected with FeLV appear to respond more slowly to treatment and may be more likely to have relapses.26 Cryptococcosis is occasionally reported in cats treated with immunosuppressive therapy or chemotherapy for malignancy.11,27 Concurrent opportunistic infections have been reported in other cats (e.g., toxoplasmosis), as well as in dogs (e.g., neosporosis or papillomatosis), suggesting underlying immunodeficiency.11,28 C. neoformans has a worldwide distribution. It primarily infects human patients who are immunocompromised, especially AIDS patients. The realized environmental niche for C. neoformans is weathered bird (especially pigeon) guano, in which it mates prolifically.29 It can also be found in decaying plant matter in hollows of certain trees.30 The organism can remain viable for at least 2 years in environments such as pigeon lofts. The majority of human cryptococcal infections result from infection with C. neoformans var. grubii (VNI). C. neoformans var. grubii is the most common Cryptococcus species isolated from dogs and cats in southeastern Australia and accounts for more than 70% of infections.22,23,25 Across the United States, most dogs with cryptococcosis are infected with C. neoformans, but infection of cats with this species is rare.11,31 C. gattii is endemic in Australia, New Zealand, Papua New Guinea, Southeast Asia, parts of Latin America, California, Mexico, Hawaii, Central and South Africa, and, to a lesser extent, certain parts of Europe (Austria, Germany, France, Italy, Greece, and Spain).32,33 Since 1999, an epidemic of infections with C. gattii has occurred in humans and domestic and wild animals in British Columbia in Canada, especially Vancouver Island, and the Pacific Northwest of the United States.34–36 The main molecular types identified in this epidemic have been molecular types VGIIa and, to a much lesser extent, VGIIb and, in Oregon, also VGIIc. C. gattii molecular type VGII can be found on living tree bark (Garry oak, maple, cedar, and pine) and in air, and has also been found in freshwater and seawater. Proximity to areas with soil disturbance was a risk factor for dogs and cats.37 In California, almost all cats are infected with C. gattii VGIII, with occasional VGII infections.11,31 The ecologic niche of this molecular type in the United States remains unclear. Approximately 25% of affected cats are housed exclusively indoors.11 In eastern Australia, 20% to 30% of human, feline, and canine cryptococcosis is caused by C. gattii,23,38 whereas in western Australia, approximately 50% of cats and dogs are infected with C. gattii.5 Most C. gattii isolates from eastern Australia belong to VGI; VGII predominates in southwestern and northern Australia.5,39 C. gattii VGI in Australia is exclusively associated with dead plant material in eucalyptus tree hollows. Cats in rural environments are at risk of infection. In contrast to C. neoformans, C. gattii isolates from the Pacific northwest and British Columbia can infect apparently immunocompetent humans.40 C. gattii molecular type VGIII infections have been reported in immunosuppressed humans in southern California.41 When disease occurs in immunocompetent hosts, it is often characterized by the presence of pulmonary mass lesions (cryptococcomas) and sometimes intracranial cryptococcomas.42 As a result, pulmonary cryptococcomas are more commonly described in human patients infected with C. gattii compared with those infected with C. neoformans.43 Based on the small size of basidiospores, the lung is thought to be the primary site of infection in humans. In cats, dogs, and koalas, the nasal cavity may be the primary site of infection (see Figure 62-1); significant pulmonary parenchymal involvement in these species is rare. Colonization of the nasal cavity may be followed by development of cryptococcal rhinitis in some (but not all) dogs and cats. However, some nasal cavity involvement may result from hematogenous dissemination, because injection of Cryptococcus spp. into the carotid artery of cats can lead to development of lesions on the bridge of the nose. Some localized cutaneous lesions may result from direct cutaneous inoculation (e.g., after a cat scratch), but skin lesions are usually interpreted as the result of dissemination or extension from the nasal cavity. The pathogenesis of cryptococcosis depends on the size of the inoculum, virulence of the cryptococcal strain, and host immunity. Cryptococcus has been aptly described as “a sugar-coated killer with designer genes.”44 Cryptococcus has several important virulence factors, including its polysaccharide capsule, laccase (which makes melanin), and other enzymes such as phospholipase, urease, and superoxide dismutase.17,42 Capsular polysaccharide is continuously shed into the host’s extracellular fluid, which includes blood, urine, and CSF. The capsule inhibits phagocytosis, depletes complement, and is well known for its ability to inhibit effective T cell responses. The ability of C. neoformans and C. gattii to grow at mammalian body temperature is also an important virulence factor and is lacking in other species of Cryptococcus. The incubation period can range from 2 to 13 months.45,46 Exposure and self-limiting infection may occur in some individuals, with reactivation of viable cryptococci in residual granulomatous foci months or years later with immunosuppressive drug therapy or illness.42 Because of this delay, a travel history to areas where Cryptococcus is highly endemic (such as British Columbia or the west coast of the United States) may be present in animals and humans residing in non-endemic regions. After infection is established within the lung or nasal cavity, the organism can spread hematogenously to other sites, particularly the lymph nodes, eye, skin, and central nervous system (CNS) (see Figure 62-1). Cryptococcus spp. has a strong tendency to invade the meninges and typically causes meningitis or meningoencephalitis. In humans, cerebral cryptococcosis is often associated with increased intracranial pressure, and this probably also occurs in cats and dogs. Spread of infection to the eye, either along the optic nerves from the brain or as a result of hematogenous spread, may result in concurrent cryptococcal optic neuritis and chorioretinitis. In animals with nasal cavity involvement, extension of infection into the brain may also follow osteomyelitis of the cribriform plate or of the ventral wall of the frontal sinus. Middle ear involvement can also occur.47 Infection also often spreads from the nasal cavity to the mandibular lymph nodes. Multifocal cutaneous involvement in cats and dogs reflects hematogenous dissemination from the primary site of infection, as do lesions in bone (e.g., digits) or periarticular soft tissues. Dogs infected with C. neoformans often develop widely disseminated disease, with involvement of sites such as the gastrointestinal system, pancreas, mesenteric lymph nodes, kidneys, myocardium, and thyroid gland (Figure 62-3).11,24,48,49 In contrast, dogs infected with C. gattii molecular type VGII in the United States tend to have isolated caudal nasal cavity cryptococcomas, which can extend through the cribriform plate into the brain (Figure 62-4). FIGURE 62-3 Ultrasound image that shows an enlarged and hypoechoic pancreas in a 7-year-old female spayed basset mixed-breed dog with systemic cryptococcosis caused by Cryptococcus neoformans var. grubii (molecular type VNI). The dog was evaluated for gastrointestinal signs that were associated with severe cryptococcal pancreatitis and enteritis. The dog’s lungs, heart, kidneys, liver, mesenteric lymph nodes, peritoneum, brain, and meninges were also involved at necropsy. FIGURE 62-4 Caudal nasal cryptococcosis in dogs infected with Cryptococcus gattii. A, CT scan showing a large caudal nasal cavity cryptococcoma at necropsy that invaded the cribriform plate and retrobulbar space in a 5-year-old Doberman pinscher infected with C. gattii molecular type VGIIa. There is a palisading periosteal response along the lateral margin of the right frontal and lacrimal bones. B and C, Postcontrast magnetic resonance images of the head of a 2-year-old intact male malamute with a 1-week history of abnormal behavior and seizures. Cryptococcus gattii was isolated from the nasal cavity. A large, contrast-enhancing mass is present in the caudal nasal cavity that invades the cribriform plate and extends into the brain. (Courtesy Dr. Stacey Hoffman.) Cryptococcosis is often a chronic infection in cats, and affected cats are usually otherwise well but occasionally have mild lethargy and inappetence. Fever is rare, and when it occurs, it tends to be low-grade. Upper respiratory tract signs due to nasal cavity involvement are common, sometimes with associated mandibular lymphadenopathy. Single or multifocal ulcerated or nonulcerated cutaneous masses may also be present (Figure 62-5). FIGURE 62-5 Multiple ulcerated skin lesions in a cat from northern California with disseminated cryptococcosis caused by Cryptococcus gattii molecular type VGIII. Other signs include peripheral lymphadenomegaly (which may be unassociated with skin lesions and is often asymmetric), lameness due to osteomyelitis or cryptococcal arthritis, and swollen digits. Rarely, involvement of the abdominal lymph nodes, kidneys, spleen, liver, thyroid, and salivary glands occur. In contrast to cats, dogs frequently develop severe disseminated disease. In one study, 80% of dogs had involvement of multiple anatomic sites, and 50% of dogs had involvement of sites other than the nasal cavity, skin, lungs, lymph nodes, kidney, eye, and CNS.11 Weight loss, lethargy, and inappetence are common nonspecific findings. Rhinosinusitis from cryptococcosis may be subclinical in dogs, so its incidence may be underestimated.11,25 However, sneezing and mucopurulent nasal discharge can also occur. Neurologic signs were present in two thirds of affected dogs from California.11 Dogs can also primarily show signs of gastrointestinal or pancreatic involvement, such as vomiting, diarrhea, and abdominal pain. Cutaneous involvement is uncommon in dogs but, as in cats, may be a marker for disseminated disease. Although rarely detected antemortem, more than half of dogs have lower respiratory involvement at necropsy.11 Other clinical signs that are infrequently present in dogs include peripheral lymphadenomegaly or lameness (due to osteomyelitis or arthritis). Upper respiratory tract signs are common physical examination findings in cats with cryptococcosis. These include sneezing; snuffling; and mucopurulent, serous, or hemorrhagic nasal discharge that is unilateral or bilateral. In some cats, a fleshy mass protrudes from the nostril. Other cats have one or more firm to fluctuant, ulcerated or nonulcerated, subcutaneous swellings over the bridge of the nose. These swellings may communicate with the frontal sinus or nasal cavity, and palpation of the lesions may reveal crepitus due to subcutaneous emphysema. Cats with nasopharyngeal cryptococcosis develop stertor, stridor, and, occasionally, otitis media. Mandibular lymphadenopathy is often present. Ulcerated or proliferative lesions of the buccal mucosa can also be found. Multifocal skin lesions consist of nodules or masses that are fluctuant to firm, up to several centimeters in diameter, and may ulcerate (see Figure 62-5). Neurologic examination abnormalities in cats with CNS involvement include obtundation; atypical behavior; slow menace, palpebral, or pupillary light responses; mydriasis or anisocoria (Figure 62-6, A); twitching or tremors; and seizures, circling, head pressing, ataxia, paresis, abnormal placing reactions, head tilt, nystagmus, and spinal pain.9 Fundoscopic examination may reveal granulomatous chorioretinitis, exudative retinal detachment, papilledema, evidence of optic neuritis, and/or retinal hemorrhage (Figure 62-6, B). Severe ocular lesions or optic neuritis may result in blindness with dilated and unresponsive pupils. FIGURE 62-6 Ocular abnormalities in cats with disseminated cryptococcosis. A, Bilateral mydriasis in an 8-year-old male neutered domestic shorthair with cryptococcal meningoencephalitis. Fundoscopic examination was within normal limits. B, Granulomatous chorioretinitis and retinal detachment in another cat with cryptococcosis. (Courtesy University of California, Davis, Veterinary Ophthalmology Service.) Many dogs with cryptococcosis are lethargic or obtunded because of disseminated disease and/or CNS involvement. When fever occurs, it is typically low grade. Other CNS signs in dogs include absent or slow menace, gag, pupillary light responses, nystagmus, mydriasis, head tilt, strabismus, anisocoria, facial paralysis, paraparesis, abnormal placing reactions, ataxia, circling, seizures, twitching or tremors, reluctance to open the mouth, absent or decreased segmental reflexes, and, commonly, cervical hyperesthesia.9,24 Some dogs show signs that resemble those of cervical disc herniation. The most common ocular abnormalities are chorioretinitis, papilledema, evidence of optic neuritis, and retinal detachment. Decreased nasal airflow may be present in dogs with nasal cavity cryptococcomas. Nodules, masses, or ulcers may involve any portion of the integument, and the nose, tongue, buccal mucosa, hard palate, lips, or nail beds. Definitive diagnosis of cryptococcosis is usually straightforward and based on evaluation of representative tissue specimens with cytology, culture, and occasionally histopathology, with or without serologic detection of cryptococcal antigen in body fluids (Table 62-1). Suitable specimens include nasal swabs, nasal washings, needle aspirates from mass lesions or enlarged lymph nodes, bronchoalveolar lavage specimens, pleural fluid, CSF, and urine. The CSF in cats and dogs with neurologic signs due to cryptococcosis may have an increased protein concentration (usually between 10 and 140 mg/dL in cats, but may be several hundred or rarely more than 1000 mg/dL in dogs).9 Leukocyte counts can range from 2 to more than 1000 cells/µL in both dogs and cats. A mixed cellular pleocytosis is generally present, with neutrophils or large mononuclear cells generally outnumbering other cell types. Some dogs have a marked eosinophilic pleocytosis. Cryptococcal yeasts are visible in most but not all animals with cryptococcal meningitis (see Figure 62-2).9 Marked deterioration in neurologic status occasionally occurs in animals with CNS cryptococcosis immediately after CSF collection. Many animals have intracranial mass lesions or increased CSF pressure, which can increase the risk of cerebellar herniation following a cisternal tap. Wherever possible, attempts to make a diagnosis with serum antigen testing or collection of specimens from other anatomic sites should be considered as an alternative to diagnosis by CSF analysis.
Cryptococcosis
Etiology and Epidemiology
Cryptococcus neoformans
Cryptococcus gattii
Clinical Features
Clinical Features in Cats
Clinical Features in Dogs
Physical Examination Findings
Dogs
Diagnosis
Laboratory Abnormalities
Cerebrospinal Fluid Examination
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cryptococcosis
Only gold members can continue reading. Log In or Register to continue