Fig. 39.1
Serial sections of mouse cerebellum prepared with the conventional fixation-dehydration method and embedded in paraffin show pCREB immunoreactivity (a–d, f, g, i, j) and DAPI (e, g, h, j) with (b, d, h, i, j) or without (a, c, e, f, g) microwave irradiation. Most of the immunostaining for pCREB is detected in nuclei of granule cells (b, large arrows), some neurons in the molecular layer (b, small arrows), and Purkinje cell s (b, large arrowheads). The microwave irradiation (b, i) cannot homogenously enhance the weak immunoreactivity (a, f). Immunofluorescence intensity of pCREB cannot be detected in the nucleoli, which are brightly stained with DAPI (i, j, arrowheads). No immunostaining is observed in immunocontrols (c, d). ML molecular layer, GL granular layer, Mw microwave treatment. Bars are 50 μm (a), 10 μm (e), and 500 μm in the large inset and 10 μm in the small inset (The figure is modified from Ohno et al. [2])
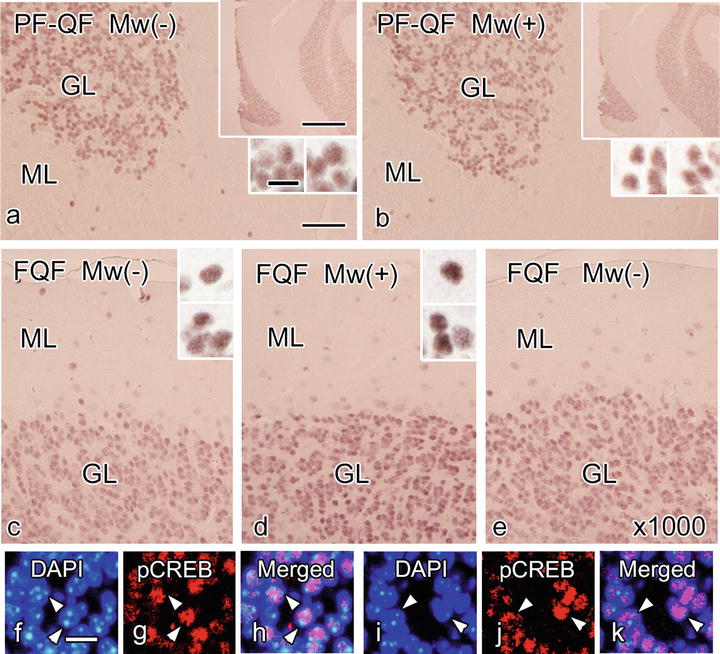
Fig 39.2
Serial paraffin sections prepared with the perfusion fixation followed by the quick-freezing (PF-QF) (a, b) or quick-freezing of fresh resected tissues (FQF) (c–k) show immunohistochemical staining for pCREB (a–e, g, h, j, k) with (b, d, i–k) microwave irradiation appeared similar to that without microwave treatment (a, c, e–h). The primary antibody was used at dilutions of 1:250 (a–d, g, h, j, k) or 1:1000 (×1000, e). The immunoreactivity with FQF is obvious even at a higher dilution (×1000) of the primary antibody (e). The pCREB immunostaining is not observed in the nucleoli, which are stained brightly with DAPI (f–k, arrowheads). ML molecular layer, GL granular layer. Nuclei are labeled with DAPI (f, h, i, k). Bars are 50 μm (a), 10 μm (f), and 500 μm (large insets) and 10 μm (small insets) (The figure is modified from Ohno et al. [2])
Masking and steric hindrance of antigens, presumably caused by the molecular cross-linkage of compact chromatin components, require antigen retrieval treatment to obtain satisfactory immunostaining, particularly when the antigens localize within the nuclear compartment or the specimens are prepared with common aldehyde fixatives [3, 4]. Microwave irradiation and other antigen retrieval techniques have been used, but are not always effective due to incompletely identified factors [5–8]. In addition, achieving full and constant retrieval effects can be difficult even under the same condition [7, 9, 10]. On the other hand, by using the cryotechniques, the pCREB immunoreactivity could be constantly enhanced [2]. Since microwave treatment did not further increase the pCREB immunoreactivity in specimens prepared with CF-FS, a similar mechanism may underlie in the antigenicity enhancement effects of CF and microwave treatment. Loosening the cross-linked proteins and increasing the penetration of antibodies are the possible mechanisms explaining the effect of the microwave antigen retrieval [7]. The immunoreactivity enhancement achieved by CF-FS would be attributable to the better preservation of immunoreactivity as well as exposure of hindered antigenicity. Tiny intracellular ice crystal s , not visible at a light microscopic level but large enough to expose some epitopes, could enhance the antigenicity by improving antibody penetration.
39.3 Analysis with FISH for the Chromosome Territory
Compared with the chemical immersion fixation followed by dehydration , morphology of normal thyroid follicles was well preserved in tissue specimens prepared by QF-FS [2]. The free tiny spaces were more prominent in the sections prepared by the conventional fixation followed by dehydration. Preservation of the thyroid morphology was not optimal in the regions with ice crystal s on the sections prepared by QF-FS [2]. The distribution of the No.18 chromosome territory in nuclei was not so clear with the immersion fixation followed by dehydration, and the distribution was unclear even when the microwave was irradiated [2]. On the paraffin sections prepared by the QF-FS, the chromosomal territory was clearly observed even without microwave treatment.
Obtaining constant results of good fluorescence labeling with FISH probes is difficult without several pretreatments such as enzymatic digestion and microwave irradiation [11, 12]. The pretreatments should be minimized to avoid tissue damages [13], and the QF-FS method could be used with fewer pretreatments for human specimens. The mechanisms of enhanced FISH signals using CF-FS without several pretreatment steps may include the increased permeability of the FISH probe, as shown in immunostaining for intranuclear antigen s [2]. A side benefit of CF-FS includes better preservation of in situ structures compared with the chemical immersion fixation -dehydration method. Therefore, QF-FS could be used to establish new criteria in the field of pathology as a method for the FISH study on paraffin -embedded human specimens with fewer artifacts .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


