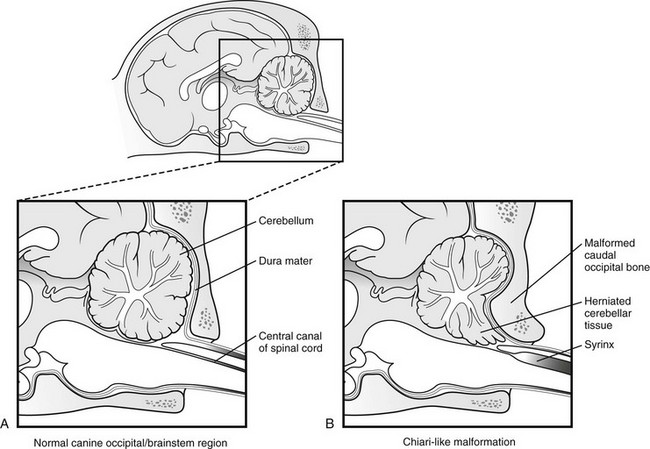
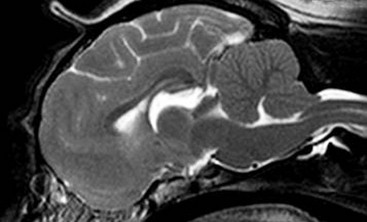
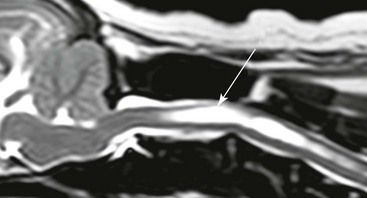
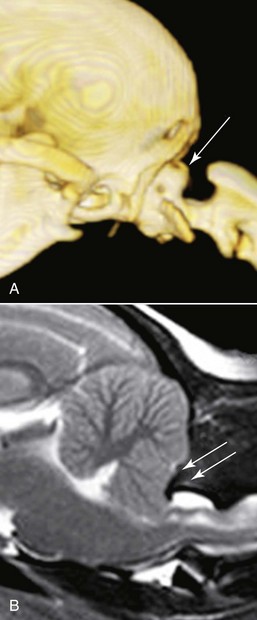
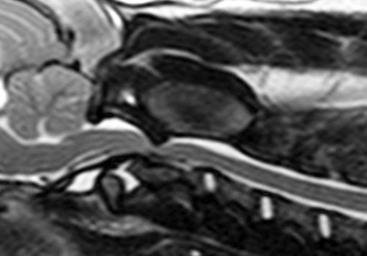
Chapter 237 Craniocervical junction abnormality (CJA) is a term that encompasses a number of developmental anatomic aberrations at the region of the caudal occiput and first two cervical vertebrae. Chiari-like malformation (CM) appears to be the most common CJA encountered in dogs, and there has been a tremendous amount of clinical investigation into this disorder in recent years. Other abnormalities in this region include atlantooccipital overlap (AOO), dorsal constriction at C1 and C2, and atlantoaxial instability. Atlantoaxial instability is discussed in detail in Chapter 235 and is not covered here. Chiari-like malformation, as noted earlier, is thought to be the canine analog of Chiari type I malformation in people. As in the human disorder, the cranial cavity is too small to accommodate the contents of the caudal fossa (cerebellum and brainstem), which results in overcrowding of the cerebellomedullary region of the brain (Figure 237-1). On MRI, the abnormality of the supraoccipital bone that causes an indentation of the caudal cerebellum often is visible. In addition, an impingement of the dorsal subarachnoid space typically occurs at the level of the cervicomedullary junction. Herniation of the caudal aspect of the cerebellum through the foramen magnum also is commonly appreciated (Figure 237-2). Most of these dogs also have cervical SM, evident on MRI (Figure 237-3). CM generally has been considered a congenital malformation of the caudal occipital region of the skull, leading to overcrowding of the caudal fossa and compression of the cervicomedullary junction at the level of the foramen magnum. However, the anatomic abnormalities associated with CM are far more complicated than simply a malformed skull in the caudal-most aspect of the occipital bone region causing a physical constriction near the foramen magnum. It is now apparent that the malformations of CM are not limited only to the caudal part of the skull. In addition, there is convincing evidence in Cavalier King Charles spaniels that there is a mismatch between the volume available in the caudal fossa region (also referred to as the caudal cranial fossa) and the parenchyma (cerebellum and brainstem) that resides within this volume; in other words, there is too much brain parenchyma in too small a space in the caudal fossa of Cavalier King Charles spaniels (compared with other small-breed dogs and Labrador retrievers). This mismatch between parenchyma and available volume also has been demonstrated in the cranial fossa (rostral and middle fossae) of Cavalier King Charles spaniels. Increased ventricular size, increased relative parenchymal volume in the caudal fossa (as a percentage of total brain parenchymal volume), and increased relative cerebellar volume all have been associated with increased likelihood of the presence of SM in the cavalier King Charles spaniel breed. Increased syrinx width also has been associated with increased ventricular size and relative caudal fossa parenchymal volume in this breed. There is some evidence in the cavalier King Charles spaniel breed that the caudal fossa volume itself often is too small compared with other dog breeds. Other anatomic abnormalities of the skull reported in dogs with SM include minute or absent frontal sinuses and abnormally small jugular foramen volumes. With regard to this latter abnormality, it is hypothesized that the constricted venous drainage from the brain caused by small jugular foramina leads to intracranial venous hypertension and increased intracranial pressure; this would lead to an increased pressure differential between cranial and spinal compartments and an increased likelihood of SM development. Figure 237-1 A, Schematic illustration of the normal shape of the caudal occipital region. B, Typical shape of this region in a dog with Chiari-like malformation. (From Dewey CW: Surgery of the brain. In Fossum TW, editor: Small animal surgery, ed 4, St Louis, 2013, Mosby, p 1438.) Figure 237-2 Midsagittal T2-weighted magnetic resonance image of a dog with Chiari-like malformation showing cerebellar herniation through the foramen magnum. Figure 237-3 T2-weighted sagittal cervical magnetic resonance image of a dog with Chiari-like malformation showing syringomyelia. In atlantooccipital overlap, the atlas (C1) is cranially displaced into the foramen magnum, and there is overlap of the occipital bone and the atlas (Figure 237-4). This displacement tends to compress the caudal aspect of the cerebellum and elevate and compress the caudal medulla (medullary kinking). AOO likely is a form of basilar invagination. Basilar invagination is a human craniocervical junction disorder in which the atlas or axis (C2), or both, telescope toward the foramen magnum. It is possible that some cases of atlantoaxial instability in dogs are also analogs of basilar invagination. Based on combined MRI and CT imaging of dogs with CJAs, there is evidence that a substantial proportion (nearly 30%) of dogs diagnosed with CM based on MRI scans actually may have AOO as the main anatomic abnormality causing compression at the cervicomedullary junction. Bone is poorly visualized by MRI, but CT images clearly delineate what bony structures are causing compression at the cervicomedullary junction. In short, it is likely that many dogs with constrictive disorders at the cervicomedullary junction that are diagnosed via MRI as having CM actually may have AOO. Figure 237-4 Midsagittal T2-weighted magnetic resonance image (A) and three-dimensional reconstructed computed tomographic image (B) of a dog with atlantooccipital overlap malformation. Similar to the AOO seen in small- and toy-breed dogs, we have seen a large number of dogs with dorsal compression at the level of C1 and C2. This compression varies in severity, with some dogs having a mild divot in the dorsal subarachnoid space and others having severe cervical spinal cord compression (Figure 237-5). At surgery, the majority of this compressive mass appears to be soft tissue, although several cases have had an obvious bony component. We believe that this disorder also may involve instability at the C1-C2 junction and possibly represents a form of basilar invagination like the AOO problem. It can occur as a sole entity or in combination with CM or atlantoaxial instability. Figure 237-5 Sagittal T2-weighted magnetic resonance image of a dog with a severely compressive dorsal lesion at C1 and C2. Syringomyelia most often is discussed in the context of CM as a causative disorder. However, SM can occur secondary to any CJA or to any disorder that disturbs normal laminar CSF flow in the subarachnoid space of the vertebral canal. Multiple mechanisms have been proposed for the formation of SM, all of which are based on the pressure differential between cranial and spinal compartments created by constriction at the cervicomedullary junction. We have found that of dogs that undergo MRI of their entire spine (not just the cervical region), most have syrinxes in the thoracic and lumbar spinal cord regions in addition to the cervical region. In a recent review of our unpublished MRI data for over 350 dogs, we found that syrinx formation begins in the cervical region and sequentially progresses to the thoracic region and finally to the lumbar region without skipping over a region. In one study of 49 Cavalier King Charles spaniels, 76% of the dogs had syrinxes in thoracic or lumbar regions or both, in addition to cervical SM (Loderstedt et al, 2011). Clinical signs in dogs with AOO typically are neck pain and varying degrees of ataxia of all four limbs. Similar clinical signs have been noted in dogs with dorsal compressive lesions at C1 and C2. It is important to realize that, especially in the cavalier King Charles spaniel breed, conditions other than CM-SM may account for some or all of the clinical signs identified. Recently it has been reported that more than 40% of cavalier King Charles spaniels with CM-SM are asymptomatic for the disorder, according to the dogs’ owners (Couturier et al, 2008); however, in a recent unpublished pilot study we found that 41% of 227 dogs demonstrated clinical signs that went unrecognized by their owners. Idiopathic epilepsy also is a prevalent disorder in the cavalier King Charles spaniel breed.
Craniocervical Junction Abnormalities in Dogs
Pathophysiology and Clinical Features
Craniocervical Junction Abnormalities in Dogs
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


