Chapter 48 Congenital and Hereditary Conditions of Camelids
The appearance of a congenital defect in any wild animal is cause for concern to keepers, zoo managers, and zoo veterinarians. Historically, congenital defects have been reported ever since animals were first maintained in captivity. Inbreeding is a constant hazard that may contribute significantly to increasing homozygosity in a small population of animals. The development of species survival plans (SSPs) in the past decade has made it possible to evaluate and scientifically share genetic lines to minimize the effects of inbreeding and linebreeding. However, challenges still exist.
Solving congenital defect problems begins with recognizing that many causes exist for congenital defects and are not necessarily hereditary (Box 48-1). Many factors may influence the well-being of the fetus, and numerous agents other than genetic factors may disrupt organogenesis.
TERMINOLOGY
Teratogenesis Process by which teratogens exert their effect.
Genotype Genetic constitution of an individual at one or more loci.
Phenotype Observable trait of an individual.
Inbreeding Breeding of related individuals.
Inbreeding depression Decrease in performance resulting from inbreeding.
Camelid All species of the camelidae.
SACs South American camelids (llama, alpaca, guanaco, vicu-a).
CAMELID GENETICS
All camelids have a diploid chromosome number of 74. There are three pairs of submetacentric autosomes and 33 pairs of acrocentric autosomal chromosomes in camelids. The X chromosome is the largest submetacentric chromosome, and the Y chromosome is a very small acrocentric chromosome. Some confusion over the classification of camelid chromosomes has arisen among investigators, perhaps because of variations in staining procedures and evaluation at different phases of meiosis.6 Chromosome banding patterns and nucleolus organizer regions have also been identified, but a complete discussion of karyology is beyond the scope of this chapter. Fertile hybrids have been produced among all four species of SACs.
TERATOGENESIS
The causes of congenital and hereditary defects are manifold (see Box 48-1). Although no specific congenital defects caused by infectious agents have been reported in camelids, it seems likely that these may occur.
Bovine virus diarrhea virus (BVDV) has caused cerebellar dysplasia, ocular defects, inferior brachygnathia, alopecia, internal hydrocephalus, and impaired immunologic competence in calves and lambs.40
Bluetongue virus (BTV) has been shown experimentally to cause central nervous system (CNS) defects (hydrocephalus, cerebral hypoplasia, dysplastic spinal cord), retinal dysplasia, and arthrogryposis in lambs. Exposure of pregnant heifers to BTV resulted in abortion, arthrogryposis, prognathia, and a “dummy-calf” syndrome.40 It is important to note that modified live virus (MLV) BTV vaccines may also exert teratogenic effects on the fetus of the pregnant ewe. The use of any MLV vaccine in any species other than those for which the vaccine was prepared is hazardous.
Both hog cholera virus and swine influenza virus are teratogenic. Feline panleukopenia virus (FPLV) causes cerebellar hypoplasia in kittens and ferrets.40 Interestingly, mature ferrets are refractory to overt infection with FPLV, yet teratogenesis occurs. In humans, examples of teratogenesis include congenital syphilitic blindness and congenital deafness from prenatal infection with German measles virus.
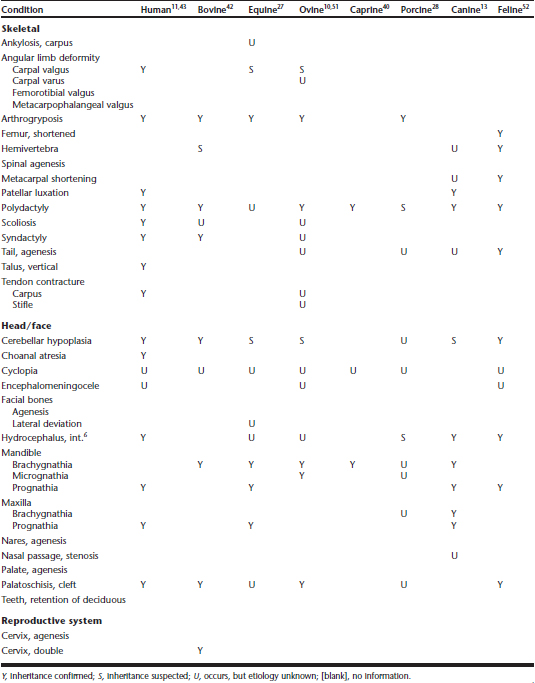
Chemically induced teratogenesis is being intensively studied in humans, livestock, and laboratory animals. No chemical teratogenic defects have been identified in camelids. However, such effects are known to occur in all other species studied, so it should be expected that chemical teratogenesis will ultimately be identified in camelids. Some congenital defects already identified in camelids are induced by chemical teratogens in other livestock species. It should be pointed out that these defects are also known to be inherited traits in one or more species (Table 48-1). Veterinarians should investigate both possibilities when congenital deformities occur.
The following general principles should be understood:
Box 48-2 lists factors necessary for teratogenesis to occur.
Poisonous plant ingestion by a pregnant SAC is an ever-present hazard to the fetus.31,32 SACs are fastidious in their eating habits, rarely consuming large amounts of strange plants, but they do investigate and try new plants. A low-dose intake may be a saving factor in camelids. Table 48-2 lists plants known to produce teratogenic defects in livestock.
Table 48-2 Plants Known to Be Teratogenic
| Genus/Species | Common Name | Animals Affected |
|---|---|---|
| Astragalus spp. | Locoweed | Cattle and sheep |
| Conium maculatum | Poison hemlock | Cattle |
| Datura stramonium | Jimsonweed | Pigs |
| Lupinus spp. | Lupine | Cattle |
| Nicotiana tobaccum | Tobacco | Pigs |
| Sorghum vulgare | Sudan grass | Horses |
| Veratrum californicum | False hellebore, corn lily | Sheep |
HEREDITARY TRAITS
Hundreds of anatomic and physiologic traits are passed from parents to offspring by gene pairing.38,44 Genome research and gene mapping have progressed at astronomical rates, but to my knowledge, a gene map of a camelid has not been produced.
Fiber coat color inheritance in llamas and alpacas has received attention by both South American22 and North American24,33,34,41 investigators, but definitive genetic studies in camelids have not been conducted and reported.
CHROMOSOMAL ABERRATIONS
A number of chromosomal abnormalities have been reported in domestic animals, but not in camelids. These defects occur during meiosis and include fusion of chromosomes, translocations of segments of chromosomes, loss of a segment, and other changes.26 The expression of the defect is determined by whether or not the change occurred on an autosomal pair or one of the sex chromosomes. Chromosomal aberrations may affect a single individual or may be perpetuated as inherited characteristics. Chromosomal aberrations may be identified by a combination of family pedigree analysis, identification of interspecific somatic cell hybrids, and cytogenetic studies, including karyotyping and various banding staining.
Genetic studies have been developed to the stage of highly technical submolecular, biochemical, and deoxyribonucleic acid (DNA) complexities. Camelid inheritance is still in the descriptive stage. Although most of the congenital defects reported in camelids are known to be inherited in one or more species of other domestic animals or humans, it is important to recognize that many may also be produced by other etiologic agents (see Box 48-1). In fact, such defects as arthrogryposis are more likely to be caused by exposure of the dam to a toxic substance at a crucial time during gestation than by genetic damage.
DETECTION OF INHERITED TRAITS
Circumstantial evidence that a congenital trait may be hereditary is based on evidence as follows6,46:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree