Chapter 9
Computed Tomography and Magnetic Resonance Imaging
The need for good anatomic information extends from the veterinary community to a diversity of basic research areas. Although detailed dissection of carcasses remains useful, suitable dissection material is not always available. While the dissection of fresh dead animals can provide a wealth of information, including identification of key landmarks, organ structure, and the overall arrangement of organ systems, the normal in vivo range of positions, shapes, and sizes of very dynamic organ systems and some vasculature cannot easily be seen in dead animals or even in “flat film” radiographs. Perhaps the most significant challenge that limits what can be learned from conventional radiography is the problem of superimposition of body parts. Within the past decade, the previously rare imaging methods of magnetic resonance imaging (MRI) and computed tomography (CT) have become much more accessible and somewhat more affordable. Recent advances in three-dimensional (3D) imaging and the availability of high-resolution diagnostic imaging tools1–2 make visualizing reptilian anatomy and pathology not only possible but also accurate, even in the most unusual species. The 3D information that each can provide may improve diagnostics by allowing for evaluation of the extent of disease and accurate identification of adjacent organ systems.3 Further, 3D imaging can guide treatment decisions and surgical solutions.4 The following discussion provides descriptions of MRI and CT, suggested approaches to maximize the quality of the information obtained, and the basic background for understanding the images. Examples of each modality are provided to illustrate the characteristics of the more common imaging types while providing baseline reptile anatomy in several taxa. Some common problems associated with imaging are also discussed and illustrated.
As is common when new tools are embraced by a field, some early foundation studies using CT and MRI in reptile medicine differ greatly in resolution or scope of technique from what is currently available.5–6 Others vary in anatomic accuracy.7–9 Caution is merited with regard to some anatomic details of early CT and MRI studies, yet their methodologies often provide sound starting points. Hence, early studies made possible many of the improved techniques, high-resolution approaches, and modifications that are in contemporary use. Additionally, studies that verify image results with associated cadaveric anatomy10 are particularly valuable when the structures are correctly identified.
Before You Start
• What is it that you need to see? Methods will differ for brain or spinal cord, air, bone, a mass (and if its consistency is important), blood flow, or fluid accumulation.
• What is the duration of the study? (Should you plan to sedate the animal or will it stay still long enough for the scan?)
• Does the animal have any metal in its body? (This may be a contraindication for MRI.)
• Will you need a contrast medium injected? (If so, be prepared to do so. The technologist is unlikely to know where the vessels are located.)
• Which plane(s) of view will be most informative? (Often, an axial series is best for high-resolution 3D reconstructions. However, a coronal (dorsoventral in a reptile) or sagittal series may also be essential to appreciate the extent of an injury or lesion.)
• What is the resolution of the images you need? (For example, small reptile patients or small structures require high-resolution scans; sequential images may be less than 1 mm apart. For larger patients, images in the series may be more than 5 mm from one another.)
• Do you need the whole animal scanned or just a part? (The time needed for the whole animal and the sizes of the data files are directly related to the volume scanned.)
• Will the scan expose the animal to excessive radiation? (For CT scans, size and resolution and machine type and age should be considered.)
• Do you need to do 3D reconstructions? If the data are to be used in a 3D reconstruction, the technologist will need to collect the data in ways that allow such reconstructions.
Addressing these questions before you start will enhance the quality of the results.
Computed Tomography
CT, or computer-assisted radial tomography, provides 3D radiographs that use thousands of fast projections taken with a radiograph tube that spins around the subject.1,11 The projections are organized as a series of two-dimensional (2D) images, similar to serial sections in a histologic study (or like slices of bread in a loaf). Computer software is used to reconstruct the 2D or 3D images of all or part of the animal. CT scans are particularly good for supplying details of structures that differ in density such as bones, eggs, scutes, or airways, and CT images eliminate superimposition of body parts. Recent advances in the machines and contrast media, as well as the software, make imaging of some soft tissues possible. Because the images are collected serially along the axis specified by the clinician, CT scans can provide flat section images that are dorsoventral, anteroposterior, or sagittal. The image data can also be assembled in 3D reconstructions2 (Figure 9-1). CT image collection is fast (usually scan durations are measured in minutes or fractions of minutes). Resolution can be quite detailed. The necessary detail needs to be addressed so that the scan provides the resolution needed. CT data can be reviewed as serial sections (Figure 9-2) or reconstructed in a variety of ways to allow for the retention of 3D landmarks (see Figure 9-1). Virtual endoscopy is possible with the use of certain imaging modalities, with or without contrast solution (oral or intravenous) and the appropriate software.
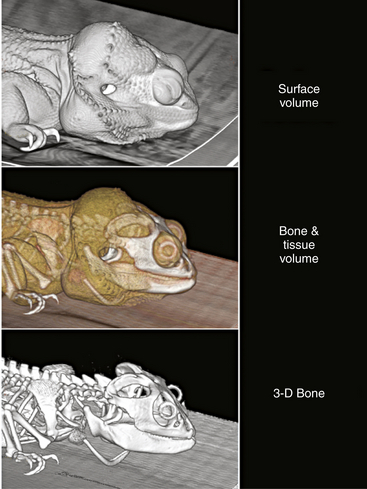
FIGURE 9-1 These lateral views of the head and torso of a Bearded Dragon (Pogona vitticeps) show the versatility of computed tomography (CT) data. They may be reconstructed in three dimensions to emphasize different structure densities and to allow understanding of external landmarks and the internal hard structures (the skeleton in this case). The same dataset from a single CT scan shows the results of three reprocessing modes. Different threshold settings allow the same dataset to be reconstructed as a surface image alone, showing the whole volume of the animal, a translucent tissue image of the whole volume with the skeleton also detected, and a three-dimensional image of the skeleton alone. Bony sutures are not seen in this image because their small size was below the level of sampling resolution. Cartilage cannot be seen when images are reprocessed for the bone but is included with other soft tissues reconstruction settings, which include some of the denser soft tissues. Bearded Dragon; snout-vent length, 22.5 cm.
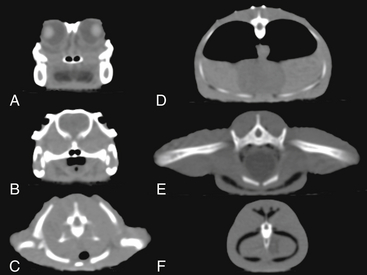
FIGURE 9-2 Two-dimensional axial computed tomographic images of several points along the body of an American Alligator (Alligator mississippiensis). At the level of the eyes (A), the jaws form the bony parts of the skull. Teeth can be seen extending from the lower jaw upward. The bony palate ventral to the choanae separates spaces that are the palatal vacuities. The vitreous and the tongue are darker. The lenses appear light. B, Posterior to the orbits, the palate is complete and the internal choanae can be seen joining the glottis. Dorsally is the oval braincase with posterior temporal bars extending ventrally and laterally. At the level of the shoulders (C), the vertebrae are mostly surrounded by the scapulae and procoracoids. The single element ventral to the trachea is the interclavicle. D, Posterior to the forelimbs, the body wall is supported by dorsal ribs and ventral ribs. The sternum is not detected. The outline of the heart can be seen ventral to the paired lungs. Two osteoderms are visible dorsal to the trunk vertebra. E, The hindlimbs are supported by robust ilia articulating with sacral vertebrae. The anterior aspect of the paired pubic bones can be seen ventrally. F, The tail vertebra and ventral y shaped chevron bone are surrounded by muscle (gray) and tendon (black). American Alligator; snout-vent length, 22 cm.
Scan data are collected in series. The shorter the distance between scans, the greater the resolution (Figure 9-3). Sequential data series are assembled to form the 3D images. Consequently, any animal movement during the exposure period will produce a movement artifact in the images. When movement occurs during a scan, usually groups of sequential data are no longer in register with one another. For example, in Figure 9-4, A, the chameleon’s tail shows a CT movement artifact; in Figure 9-4, B the turtle’s anterior trunk shows a movement artifact due to breathing. If the area that moved is not part of the key study site, the scan may still be useful. However, for the most reliable scan quality, general anesthesia may be required.
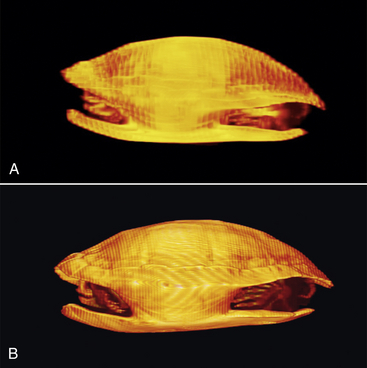
FIGURE 9-3 Image resolution of this Redear Slider (Trachemys scripta) CT improves with decreased spatial intervals. A, The sequential sections were three times farther apart than in (B). B, Details of the skull, digits, and caudal vertebrae can be identified in this higher resolution scan. Redear Slider; straight carapace length, 24 cm.
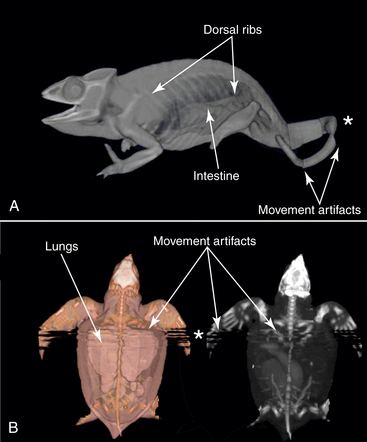
FIGURE 9-4 This set of three-dimensional (3D) computed tomographic (CT) images shows movement artifacts. Chameleons (Panther Chameleon [Furcifer pardalis]) have thin bones and large saclike lungs; both can be imaged with CT scans. These 3D reconstructions show both large areas of the body filled by the low-density field and the chameleon’s numerous ribs that extend the entire length of the trunk. The dorsal ribs are jointed at their articulations with the spine, and with the ventral ribs (gastralia) from the belly. A, The data were reconstructed from 0.6-mm serial images and reprocessed so that both skeletal and airway data could be seen. Panther Chameleon; snout-vent length, 12.6 cm. B, Postprocessing of the turtle image data with false color highlights some skeletal and soft tissue structures such as lungs and parts of the intestine (left) that do not appear when the same dataset is reconstructed as a maximum intensity projection (MIP) 3D image (right). Movement artifacts (∗) appear as discontinuous segments in the image associated with tail movement by the Chameleon (A) and breathing by a neonate Leatherback Sea Turtle (Dermochelys coriacea) (B). Straight carapace length, 6.2 cm.
Vasculature and organ systems (particularly hepatic, renal, and cardiopulmonary) may be visualized with injection of a radiopaque contrast medium. Many of the injection media currently available are of sufficiently low viscosity that they are suitable for small subjects. Viscosity of the contrast medium is important because of the small sizes of needles or cannulae used to deliver the solution. There are no systematic studies focused on identifying optimal dosages of CT contrast media in reptiles. A dose that is 25% to 35% of the human pediatric concentration of iopromide contrast medium (Ultravist Injection 370 mgI/mL at 1 to 2 mL/kg) can be used. The package information indicates that the maximum dose of Ultravist injection should not exceed 4 mL/kg.12 This dose should provide adequate contrast for cardiac, renal, and vascular imaging in turtles and iguanas. Similarly, good results were obtained with an iodixanol contrast medium (Visipaque 270 [550 mg iodixanol/mL], GE Healthcare Inc., Princeton, NJ; in doses of 0.1 gI/kg body weight) in a Loggerhead Sea Turtle (Caretta caretta). Other published studies that used other intravenous or enteric contrast material or higher doses had varied success.6,10 A recent review13 summarizes CT contrast media, their characteristics, and their effectiveness for various applications in small animals. Although focused mostly on mammals, it provides a good overview and perspective.
A numbers of diverse reptilian studies using CT now have been published.10,14–22 These studies represent both novel and traditional uses of the imaging tools. For example, the techniques used to diagnose disease in extant reptiles are now being applied to understand diseases of archosaurs that have long been extinct.14–15 CT studies of modern reptiles and birds (“avian reptiles”) are used commonly to understand the structure and function of fossilized avian and nonavian dinosaurs.15 These various studies highlight the utility of CT in understanding both normal anatomy and pathology, and they put the historical occurrence of reptilian disease in perspective.14,17–22
Magnetic Resonance Imaging
All the MRI images included in this chapter were collected with a closed MRI. For large-bodied animals, the donut-shaped magnet and a receiver are housed together. For smaller animals or parts of the animal, smaller receivers, termed coils, may be used to provide clearer images by increasing the signal-to-noise ratio (Figure 9-5) and reducing artifacts. Head, knee, or wrist coils usually provide good quality images when the length of the animal or the region of interest is not great. Animals that move little when handled may need just minimal restraint. Others may be briefly sedated with a nonvolatile anesthetic. No metal can be on or in the animal because it may heat up during imaging and can cause discomfort or burns. Additionally, the signal from metals may leave large artifacts in the image, often obscuring the site of interest.
FIGURE 9-5 The closed magnetic resonance imaging machine has a donut-shaped magnet as its largest component. It produces, sends, and receives signals. For small-bodied animals like this turtle, a smaller receiver was mounted inside the gantry to provide better images.
Because the MRI imaging is based on the responses of fluids, fats, or circulatory characteristics (e.g., flow rates),1,6 this imaging modality is good for understanding the forms and positions of soft tissues, tracking changes, and identifying pathologic conditions. The pictures supply detailed landmarks and can provide an understanding of normal positions and sizes of soft tissues, such as the gastrointestinal tract, liver, and lung. MRI is also a good imaging choice for assessing neurologic conditions such as spinal cord injury, ischemic injury, or hydrocephalus. It is particularly effective for assessing the age of a lesion, tracking acute versus chronic conditions, extent of edematous change, assessing joint structure, damage, or disease, and tracing blood flow.
MRI is based on a set of techniques that uses the different biochemical and biophysical properties of the tissues in response to the magnetic pulses to produce images. An MRI machine focuses a magnetic field that rapidly spins around the subject.1 Protons in tissues or liquids temporarily respond in tissue-specific ways to generate an image. Images are composed of a series of 2D images or “slices” that may be viewed alone or in 3D reconstructions.
The images can be collected in many different ways. For basic needs, two major categories of imaging modes can be used: T1-weighted and T2-weighted studies (Figure 9-6). The terms refer to the images that result from tissue-specific biophysical properties of protons as they respond to magnetic fields. Often T1 scans are described as anatomy scans and T2 scans are pathology scans. Many special techniques for specific applications are based on variations of these two main types of imaging modalities and the tissue response characteristics that they emphasize. For those interested in the physical principles of MRI, several printed and online resources are available, and MRI machine characteristics are available at imaging facilities. For the purposes of this chapter, the essential difference is that, in T1 images, fat is bright, fluid (i.e., flowing blood, edema, cerebrospinal fluid [CSF]) are dark, and solid masses and cortical bone are dark. Bone marrow, because of the fat within, appears bright. T1 techniques are good for imaging brain and spinal cord because of the high levels of lipids in these regions. T2 techniques show fluids such as flowing blood and cysts as bright areas; pooling blood usually is dark gray, fats appear gray, and bone is dark. T2 imaging is good for visualizing circulation.1 Several publications demonstrate the advantages of each of these two imaging modalities in diverse examples of reptiles.23–27
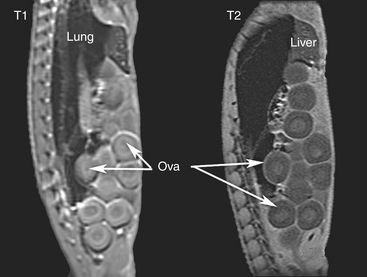
FIGURE 9-6 Two sagittal magnetic resonance images of a female Green Iguana (Iguana iguana) torso taken at roughly the same position. Cranial is toward the top of the image. In the T1 image (left), fat is bright, air is black, fluid (i.e., blood, edema, cerebrospinal fluid [CSF], or yolk) is dark, and cortical bone is dark. Parts of the spinal nerves can be seen as white dots running along the spine on the left side of the image. In T2 images, fluids are bright, fats are gray, and bone is dark. The posterior trunk vertebrae and vertebral muscles are easy to identify. This T2 scan (right) caught little blood flow (seen immediately to the left of the three more anterior ova). The more liquid centers of the yolked ova are clear in both images.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


