Chapter 21
Complications
Abnormal or laboured breathing
Expansion capabilities of different colloids
Adverse effects of colloid administration
Blood product transfusion triggers
Intravenous lipid emulsion (ILE)
Introduction
The layout of this chapter is based on identification of an abnormal behaviour or measurement in the perianaesthetic period, such as an arrhythmia, cyanosis, hypotension, or laboured breathing. Sometimes the cause of the abnormality is immediately obvious, such as hypotension following injection of an anaesthetic agent or change in patient body position, other times it is necessary to work through a checklist to identify the origin of the abnormality. Potential causes are listed for abnormal signs and have been classified according to categories to aid differential diagnosis. The following eight categories comprise a good checklist when troubleshooting a patient’s problem: Drugs, Equipment, Mechanical, Other patient problems (than cardiac or respiratory) and procedure, Cardiac, Respiratory, Allergic, and Toxic. The first letter of each category forms the acronym DEMOCRAT, which is helpful to ensure a thorough and systematic evaluation of the patient is performed. Suggestions for management of each cause are included and further explanations are presented in the following boxes and text. Details of events leading up to cardiac arrest and the current approach to cardiopulmonary cerebral resuscitation (CPCR) are given in the next chapter. Hypothermia and hyperthermia are covered in Chapter 2. Regurgitation is discussed in Chapter 15 and the ruminant chapters. Myopathy and neuropathy that may occur after anaesthesia in horses is discussed in Chapter 11.
Abnormal breathing
An animal’s breathing pattern during anaesthesia is not the same as in the conscious state, varies between species, and may be influenced by the specific anaesthetic agent administered. The breathing pattern may also be changed by the patient’s current health status, acid–base status, depth of anaesthesia, or other conditions that may develop during anaesthesia.
Abnormal or laboured breathing
Breathing that is gasping, jerky, shallow, or has excessively large thoracic excursions, flexion of head and neck, and/or tracheal airflow that is zero, reduced, or increased, is usually associated with abnormalities that may have serious consequences for the patient (Table 21.1, Boxes 21.1–3).
Table 21.1
Abnormal or laboured breathing: causes and management
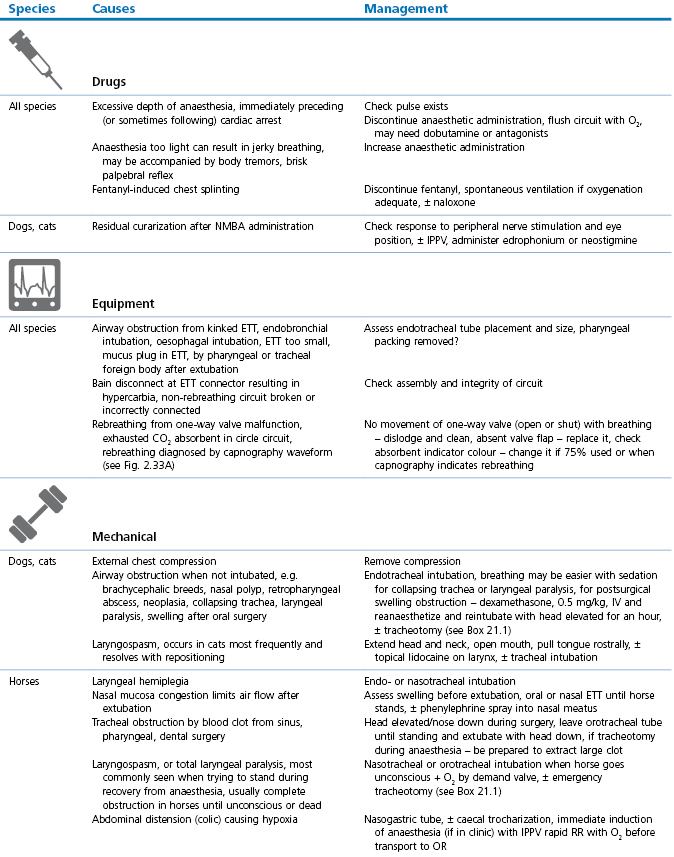
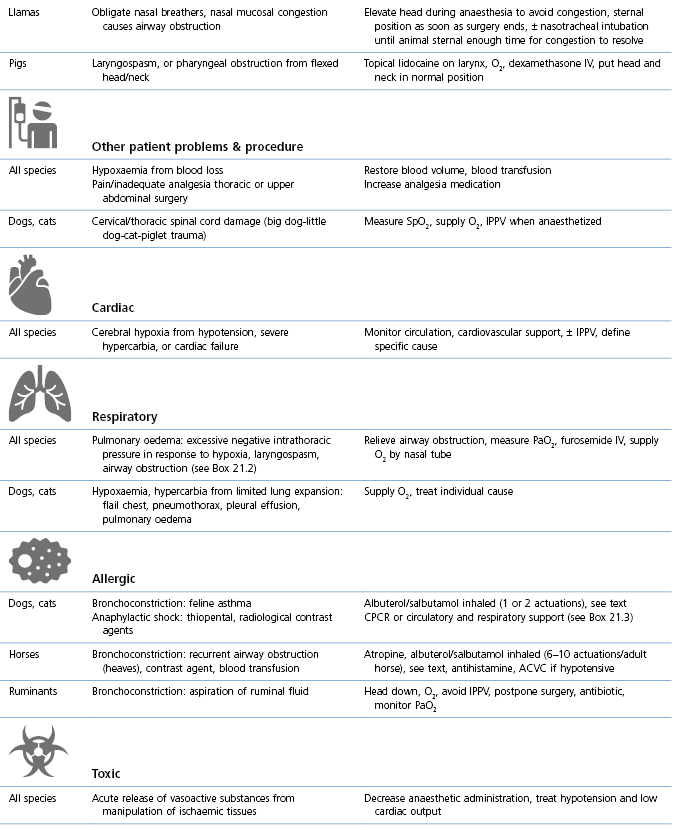
NMBA: Neuromuscular blocking agent; ETT: endotracheal tube; OR: operating room/theatre, CPCR: cardiopulmonary cerebral resuscitation (see Chapter 22).
Bronchodilation
Albuterol (USA name) is the same as salbutamol (International name) and is administered by inhalation for its direct β2-adrenergic agonist effect on bronchial smooth muscle as rescue medicine for bronchoconstriction in humans. The stated time of onset is 5–20 minutes. There are several products commercially available and Ventolin® is the only product that does not contain ethanol as a co-solvent. Albuterol/salbutamol has been used to treat horses with recurrent airway obstruction (RAO, ‘heaves’) that have increased airway resistance and decreased lung compliance from bronchoconstriction, airway wall oedema and mucus accumulation in small airways (Derksen et al., 1999; Bertin et al., 2011). Measurements of pulmonary function determined that 360 µg of aerosolized albuterol/salbutamol in conscious horses with RAO caused significant bronchodilation and that increasing the dose to 720 µg did not significantly enhance bronchodilation or increase the duration of action (Derksen et al., 1999). Onset of bronchodilation was rapid within 5 minutes and the effect lasted 0.5–3 hours. Another experimental study (Bertin et al., 2011) administered albuterol (Ventolin) by metered-dose inhaler that delivered 90 µg albuterol/actuation (puff) in 10 conscious horses with RAO. Measured changes in transpulmonary pressures and lung compliance confirmed that the average dose of albuterol/salbutamol required for maximal bronchodilation in the horses studied was 6 actuations (540 µg) but that there was a large variation between horses, with maximum bronchodilation achieved between 2 (180 µg) and 10 (900 µg) actuations.
Administration of albuterol/salbutamol may be an effective treatment of hypoxaemia in anaesthetized horses. In a clinical study, horses anaesthetized for surgery for colic were administered albuterol into the Y-piece of the circle breathing circuit during inhalation at a dose rate of 2 µg/kg (Robertson & Bailey, 2002). Arterial blood collected 20 minutes later revealed a significant increase in average PaO2 from 8.25 kPa (62 mmHg) to 15.8 kPa (119 mmHg). The bronchodilation may have improved ventilation in underventilated parts of the lungs and thereby increased O2 uptake. No significant changes in heart rate (HR) or blood pressures were recorded. A dose rate of 2 µg/kg (equivalent to 10 actuations for a 450 kg horse) is higher than the requirement for bronchodilation measured in conscious horses, and it may be that less albuterol/salbutamol will produce satisfactory results in some anaesthetized horses.
Bronchoconstriction is also a component of an allergic or anaphylactic reaction. Albuterol was administered as part of treatment to one horse that developed respiratory failure in response to injection of contrast media during imaging (Gunkel et al., 2004). The response to albuterol was minimal, although subsequent injection of atropine, also a bronchodilator, produced a slight positive effect. The clinicians involved with this horse were persistent with their heroic efforts despite an extremely low PaO2 of 4.26–5.2 kPa (32–39 mmHg) that increased to 5.6 kPa (42 mmHg) after the horse was standing. Treatment included albuterol/salbutamol, atropine, furosemide, and O2, and the horse survived to be reanaesthetized without complications one month later.
Albuterol/salbutamol has been studied in healthy cats with drug-induced bronchoconstriction using measurements of lung function by whole body plethysmography (Leemans et al., 2009). One puff from a metered-dose inhaler (Ventolin®) induced a significant antispasmodic effect lasting for 4 hours. In an emergency situation in cats with asthma, further doses may be given, such as an additional dose every 30 minutes for up to 4 hours (Padrid, 2006).
Tachypnoea or panting
Tachypnoea or panting may result in hyperventilation or hypoventilation depending on the adequacy of alveolar ventilation. Panting is a common side effect of opioid administration in dogs, most frequently seen with morphine, oxymorphone and hydromorphone and less frequently with butorphanol and buprenorphine. Rapid respiratory rates may be seen, especially in small dogs, during inhalation anaesthesia. Small ruminants have a relatively fast respiratory rate that generally decreases with increasing depth of anaesthesia. α2-Agonist sedative administration to healthy or febrile horses occasionally causes short-lived increases in respiratory rates to 40–100 breaths/min, with minimal decreases in PaCO2. No specific treatment is recommended for the tachypnoea.
Differential diagnoses of a faster than normal respiratory rate include:
•Hyperthermia from excessive application of external heat, anaesthetic-induced impairment of thermoregulation, bacteraemia, or malignant hyperthermia.
Opioid-induced panting is usually controllable by intermittent positive pressure ventilation (IPPV). Depth of anaesthesia should be assessed using the known amount of anaesthetic agent administered and observation of eye signs and reflexes. The anaesthesia machine and circuit should be checked for faults and corrected. Hyperthermia should be treated by removal of external heating devices and application of ice packs if considered necessary. Malignant hyperthermia is usually differentiated from other forms of hyperthermia by the production of excessive amounts of CO2 seen as a rapid increase in indicator colour and temperature of the CO2 absorbent, tachycardia, hypertension, and a pH and blood gas analysis confirming respiratory and metabolic acidoses (see Chapter 2).
Irregular breathing patterns
Some irregular breathing patterns observed during anaesthesia indicate respiratory centre depression but may be characteristic of a particular anaesthetic agent, such as ketamine. Some breathing patterns occur frequently and are considered to be a ‘normal’ abnormality. Irregular breathing is an indication that respiratory control is impaired and that fluctuations in PaO2 and PaCO2 are probably occurring.
•Apneustic: This pattern has prolonged end-inspiratory pauses of several seconds duration where the lungs remain inflated longer than usual. This pattern is commonly associated with ketamine anaesthesia and can be observed in cats and horses anaesthetized with ketamine.
•Ataxic breathing: Both rhythm and depth of breathing are irregular.
Arrhythmias
Causes and management
This section covers some of the most common arrhythmias detected during the perianaesthetic period (Table 21.2). Second degree AV block is a common finding on preanaesthetic evaluation of conscious horses and often develops in all species after administration of α2-agonists. Premature ventricular complexes (PVC) are a common consequence of myocardial ischaemia in dogs with gastric dilation-volvulus, splenic neoplasia, and thoracic contusions acquired during a road traffic accident. PVCs occur commonly in dogs for several minutes after induction of anaesthesia with thiopental, often as a bigeminal rhythm. PVCs may occur during halothane anaesthesia in dogs and cats with hypercarbia from hypoventilation. They are not a common occurrence during anaesthesia in other species.
Table 21.2
Some electrocardiographic abnormalities occurring during anaesthesia; causes and management
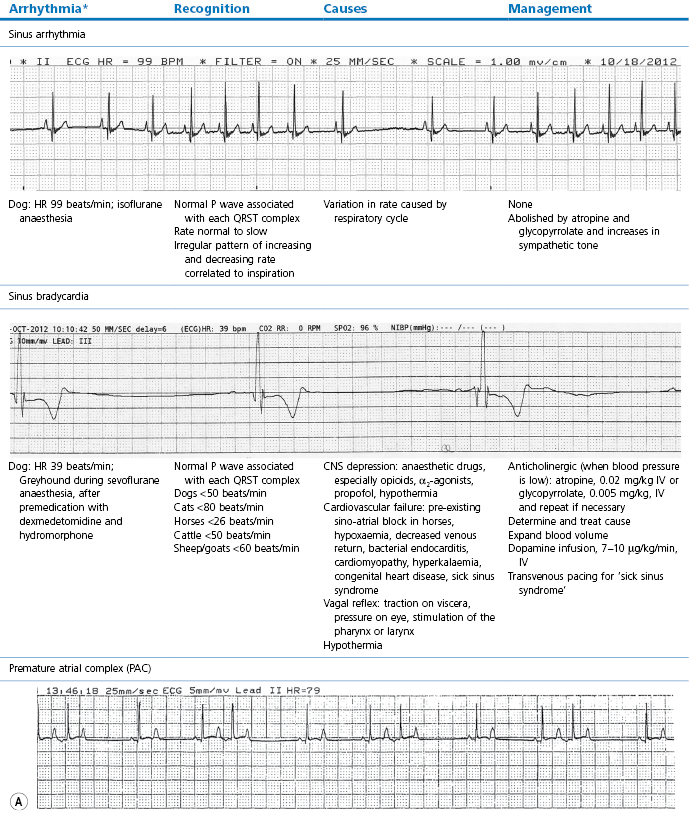
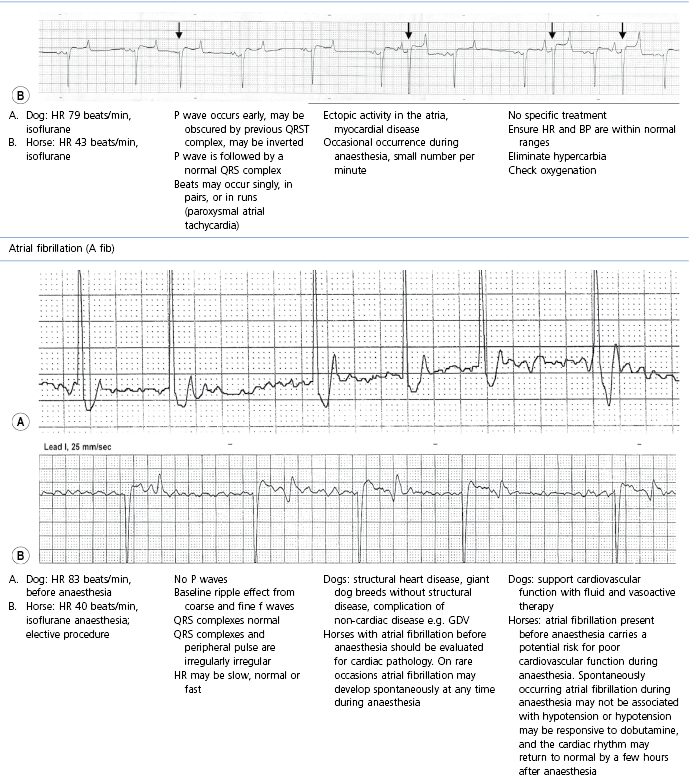
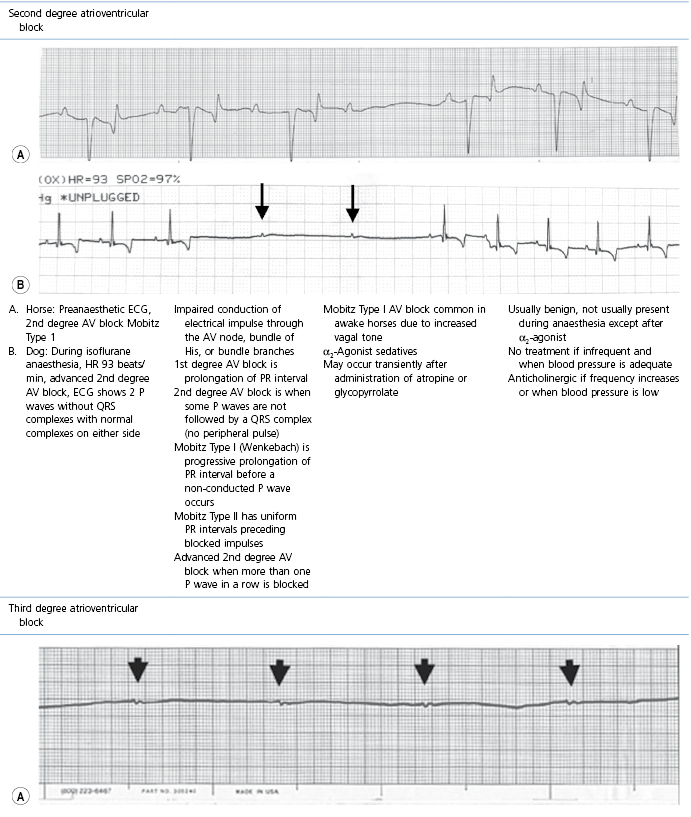
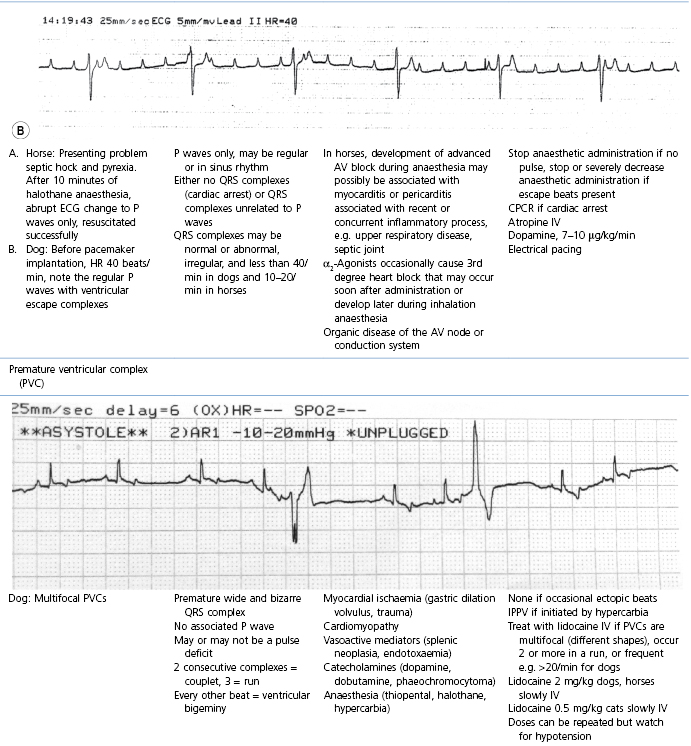
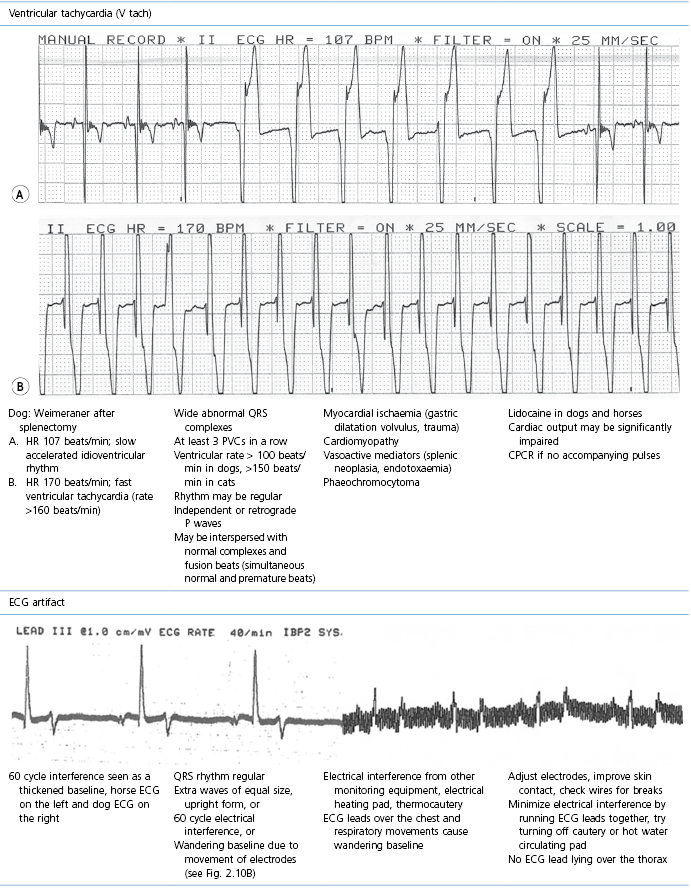
* Configuration of the ECG will depend on the Lead that is recorded. Commonly, during anaesthesia standard Lead II is used for cats, dogs, pigs, and small ruminants, and a base-apex Lead I for horses. Equine atrial premature complex, equine atrial fibrillation, and canine third degree AV block ECGs courtesy of Dr Jennifer Adams.
Tachycardia
Tachycardia denotes a rapid heart rate, with the rate exceeding 180 beats/min in dogs, 240 beats/min in cats, and 50 beats/min in horses (Physick-Sheard, 1999; Carr et al., 2001). Sinus tachycardia is regular and continuous. When the rhythm originates outside the sinoatrial node (atrial tachycardia), the P waves may be a different shape from normal but the QRS complexes are normal. Tachycardia may also be paroxysmal.
Ketamine, atropine, or glycopyrrolate injected IV frequently cause a fast heart rate in dogs and cats that is close to but does not usually achieve the strict definition of tachycardia. Hypoxaemia, and sometimes hypercarbia, cause increased heart rates. Excessively increased heart rate does not usually accompany hypotension induced by deep anaesthesia or inhalant agents. Heart rate may not change during blood loss in the anaesthetized animal until blood volume is moderately to severely depleted, but will generally occur in response to an abrupt severe decrease in blood pressure caused by anaphylactic reactions. Heart rates may increase when analgesia is inadequate during surgery. A heart rate increased above that normally expected for the anaesthetic protocol can be caused by ruminal distension (bloat) in ruminants, gastric distension in horses, or urinary bladder distension in dogs. Tachycardia due to endotoxaemia during anaesthesia of horses with colic has been associated with increased mortality. Endogenous (phaeochromocytoma) and exogenous catecholamines (administration of dopamine, dobutamine or ephedrine) can cause tachycardia or fast heart rates.
Clearly, management varies depending upon the cause of the fast heart rate. The effects of ketamine and anticholinergics generally wane within 20 minutes. Hypoxaemia and hypercarbia should be corrected. Blood pressure should be measured and treated if low. Tachycardia may occur in response to haemorrhage and low blood pressure following recovery from anaesthesia when the baroreceptor reflex is no longer depressed by the anaesthetic agents. Intra-abdominal haemorrhage after castration or laparotomy is an example where tachycardia is present but bleeding is not apparent. Observation of mucous membrane colour, CRT, and pulse pressure, and ultrasound or percutaneous needle aspiration of the abdominal cavity are required for the diagnosis of haemorrhage.
Pain should be managed appropriately. If feasible, a stomach tube should be passed in a small ruminant to decompress the rumen and the patency of a stomach tube in a horse should be checked. The presence of urinary retention can be assessed by palpation, ultrasound, or a walk outside for a dog after recovery from anaesthesia. Tachycardia and hypertension caused by release of catecholamines from a phaeochromocytoma should be managed by infusion of nitroprusside. For dogs, 25 mg of nitroprusside added to 500 mL saline produces a concentration of 50 µg/mL (a smaller volume or more dilute solution can be made up for small dogs). The dose to calculate for this clinical problem is 1 µg/kg/min, however, half that rate may be sufficient to treat hypertension and the infusion should be stopped or reduced before reaching the target mean arterial pressure (MAP) otherwise an overcorrection will occur. Catecholamines may be released from a phaeochromocytoma in surges resulting in rapid swings in blood pressure, therefore, the infusion rate must be tightly controlled and blood pressure must be continually monitored to facilitate immediate changes in nitroprusside infusion rate in response to changes in blood pressure.
Cyanosis and hypoxaemia
Cyanosis is the bluish colour of skin or mucous membranes caused by the presence of more than 5 g desaturated haemoglobin per 100 mL of blood. The blue mucous membrane colour may be difficult to detect in an anaemic patient. The colour of mucous membranes may not accurately reflect haemoglobin O2 saturation in the presence of excessive vasodilation or vasoconstriction.
Causes and management
Management involves administration of O2 and correction of the initiating cause (Table 21.3, Box 21.4). Consequences of hypoxaemia depend on the timing and effectiveness of intervention and may range from death during anaesthesia to blindness after anaesthesia.
Table 21.3
Cyanosis or SpO2 <90% (hypoxaemia): causes and management
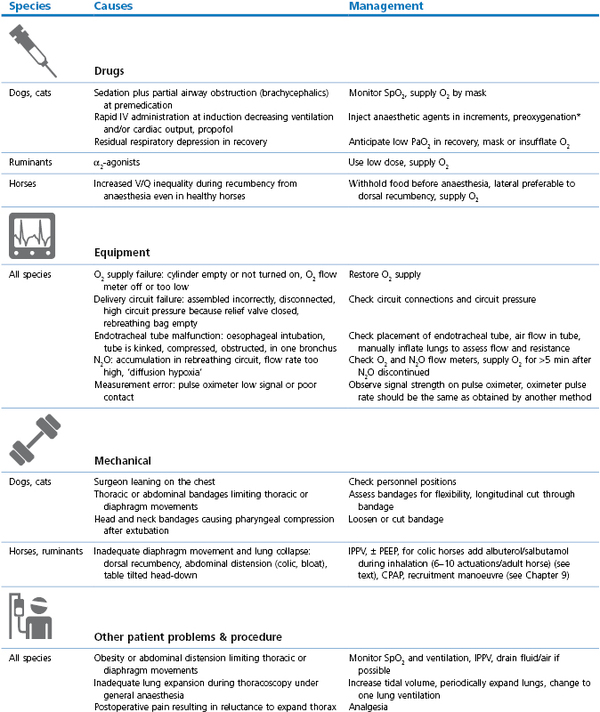
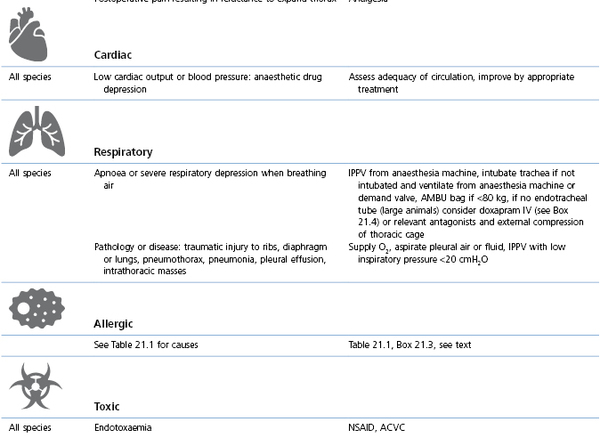
* Preoxygenation: administration of O2 by mask for a few minutes before and during induction of anaesthesia; V/Q: ventilation/perfusion inequality within the lungs; PEEP: positive end-expiratory pressure (see Chapter 9); CPAP: continuous positive airway pressure (see Chapter 9); CPR/CPCR: cardiopulmonary cerebral resuscitation (see Chapter 22); ACVC: circulatory support (see Box 21.5).
α2-Antagonists
Administration of an antagonist may be life saving for an animal in a life-threatening situation. However, the consequences of sedative (or opioid) reversal must be considered. α2-Agonist sedatives provide profound sedation and drug and dose-dependent analgesia and may play a large part in maintaining anaesthesia. Abrupt reversal can result in the patient becoming suddenly awake and trying to stand up, unmask ketamine resulting in CNS excitement, or cardiac arrest as the patient suddenly experiences pain and a surge of catecholamines is released. If there is time, administration of an antagonist should be incremental over a period of minutes, allowing progressive assessment and control of the animal.
Yohimbine is a competitive α2-receptor antagonist. It is licensed as an antagonist to xylazine in dogs and has been used in horses but may cause a serious adverse reaction, including death, in this species.
Tolazoline is a competitive α1– and α2-receptor antagonist. In some countries, it is marketed for reversal of xylazine sedation in horses and is an effective reversal agent for xylazine in cattle. Measurement of the decline in blood concentration has led to the recommendation of a cattle slaughter withdrawal time of 8 days and withholding of milk for 48 hours (Hsu, 2008b). Adverse side effects of tolazoline administration are tachycardia and hypotension, thus it is advisable to administer tolazoline slowly over several minutes to avoid cardiovascular collapse. When tolazoline is used to decrease ataxia attributed to xylazine after total IV anaesthesia in horses, as opposed to an emergency situation, a low dose of 0.8–1.0 mg/kg IV provides a satisfactory effect.
Tolazoline has also been evaluated for effectiveness of antagonism in horses sedated with detomidine, 0.02 mg/kg, IV (Hubbell & Muir, 2006). Although tolazoline did not produce complete reversal, time to recovery was shortened, ease of walking was improved, and ataxia minimized. The horses appeared to be nervous for 15 minutes after reversal. Administration of tolazoline, 4 mg/kg, IV 20 minutes after horses were sedated with detomidine, 0.04 mg/kg, IV decreased sedation, head position returned to normal, and cutaneous analgesia was lost (Carroll et al., 1997). No effect of tolazoline on HR, MAP, respiration rate (RR), or temperature was recorded. Use of tolazoline and atipamezole in cattle has been described in Chapter 12.
Atipamezole is licensed as an antagonist to medetomidine and dexmedetomidine sedation in dogs to be dosed IM as an equal volume as the volume of sedative administered, equivalent to a dose of atipamezole five times that of medetomidine or dexmedetomidine (see product package information sheet). Various dose rates of atipamezole antagonize detomidine sedation in horses (see Chapter 11). Sedation provided by a continuous infusion of detomidine in standing horses for laparoscopy was reversed within 10 minutes of injection of atipamezole given at a dose equal to the cumulative dose of detomidine for each horse (van Dijk et al., 2003). No relapse into sedation was noted. A case report has described the use of atipamezole to treat detomidine overdose. A pony received a 10-fold overdose of detomidine (total 0.3 mg/kg) with ketamine, a mistake that was recognized when the pony failed to recover at the expected time (Di Concetto et al., 2007). Increments of atipamezole injected over 30 minutes reached a dose (1.1 mg/kg) that was 3.6 times the dose of detomidine and decreased sedation enough for the pony to stand. In experimental investigations of horses sedated with detomidine, 0.01 or 0.02 mg/kg, IV, atipamezole, 0.1 mg/kg, IV produced incomplete antagonism but significant improvement in locomotion (Buchner et al., 1999; Hubbell & Muir, 2006). The degree of antagonism was less than obtained after tolazoline administration (Hubbell & Muir, 2006).
Hypotension
Extreme changes in clinical signs, such as white mucous membranes (hypotension or hypertension), slow heart rate, or lack of peripheral arterial pulse, clearly warn about the need for intense, aggressive treatment. Of concern is the fact that these parameters may be within the normal ranges and yet the cardiovascular system may need support. For example, heart rate may be normal but the arterial blood pressure too low, or mucous membranes may be pink but the blood pressure too low. Except for the extreme values, evaluation of several parameters is necessary to assess accurately cardiovascular status of anaesthetized patients.
Mean arterial pressure in awake, healthy adult dogs and cats is 90–100 mm Hg (systolic pressure 135–160 mm Hg, diastolic pressure 65–80 mm Hg), MAP in conscious horses is 95–115 mmHg, and in sheep 90–110 mmHg. Hypotension is MAP ≤60–65 mm Hg or systolic pressure ≤80 mmHg. Other clinical signs are:
•Prolonged CRT, blood oozing at the operative site rather than free flowing
•ST segment slur on the ECG indicating myocardial hypoxia
•Dysrhythmias may cause hypotension
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



