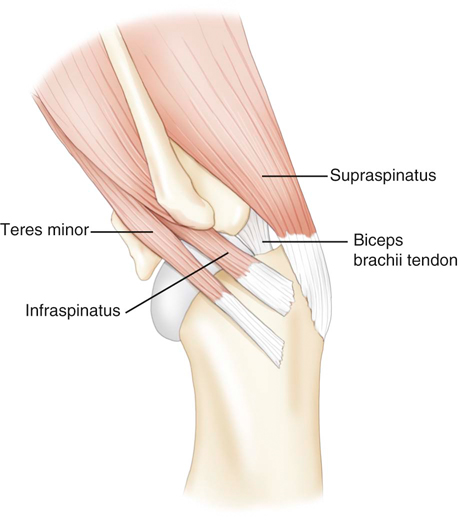
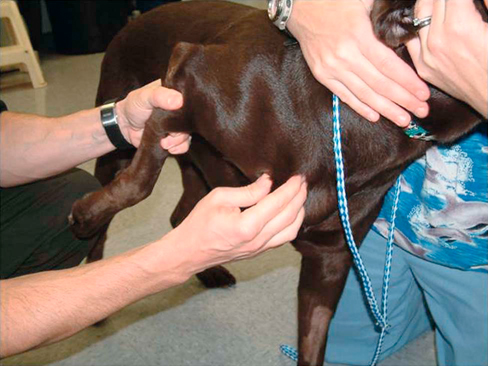
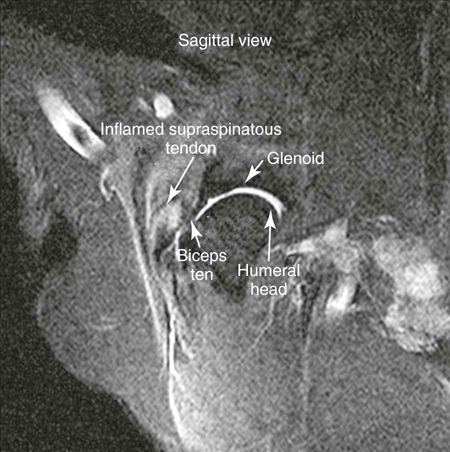
Muscle strains are the most frequent injury in human sports and can be minor, with tearing of a few muscle fibers with pain and local spasm, to complete muscle rupture.1 Acute muscle injuries rarely have been reported in the canine veterinary literature, and discussion of chronic muscle disorders in dogs is limited to a handful of classic syndromes or inflammatory conditions. However, the reports of muscle strains in human sports medicine are bountiful and well documented in terms of evaluation and treatment.2–4 It seems likely, given the similarities of the human and canine musculoskeletal system, injuries common in the human athlete would be common in their canine athlete counterpart. The low reported prevalence of muscle injury in dogs is probably owing to a failure to diagnose the condition. Inflammation and edema play a large role in muscle strains. Supportive care to treat the inflammation and swelling is often provided; however, in most cases little thought is given to the damage done to the involved muscles and their subsequent rehabilitation. The strength of muscle contraction may be greatly affected by even a minor strain injury. Some muscle fibers heal, but normal microscopic structure is not restored and scar tissue persists.1 Healing of the muscle by scar tissue predisposes it to reinjury and possibly to muscle contracture or permanent shortening.1 Platelet-derived growth factor (PDGF) Transforming growth factor-B1 (TGF-B1) Transforming growth factor-B2 (TGF-B2) Vascular endothelial growth factor (VEGF) Basic fibroblastic growth factor (bFGF) In addition, PRP contains factors that include antibacterial and fungicidal proteins, metalloproteinases ADP, ATP, calcium ions, histamine, serotonin and dopamine, that may also affect tissue inflammation.9 Stem cells, specifically mesenchymal stem cells (MSC) found in bone marrow and adipose tissue, can differentiate into multiple cell lines including bone, cartilage, and fibrous connective tissue.10 In addition, MSC secrete cytokine and growth factors that reduce inflammation, inhibit programmed cell death of the cells within the tissue, recruit circulating stem cells to the area, and integrate and reform tissue.11 Synergy between stem cells and PRP has been reported.12–18 Certain growth factors and cytokines released from platelets bind to receptors on the surface of the stem cell and initiate a cascade involving signal transduction, gene expression and stem cell proliferation. PRP also provides a delivery vehicle or scaffold to support cell survival and proper differentiation.19 Most forelimb injuries sustained by performance dogs involve the shoulder. The shoulder joint is able to withstand large forces and provide extraordinary mobility while maintaining the stability and control necessary to enable precise function of the forelimb during activity. More than 25 muscles are required for shoulder motion, which involves components of flexion, extension, rotation, abduction, and adduction.20 Given the muscular contribution and complexity of this intricate network of interlinked support mechanisms, the potential for muscle injuries causing performance issues and lameness is significant (Figure 33-1). When muscle strains occur during activities, muscle fibers can be disrupted, most commonly near the muscle-tendon junction.20 These injuries are typically characterized by initial inflammation and pain, followed by healing with marked scar tissue, impeding the ability of the tendon to stretch.21 Muscle strains have been reported in every major muscle group of the forelimb, but the muscles at particular risk in performance dogs are the biceps brachii and the supraspinatus. One of the most common shoulder conditions seen in performance dogs is bicipital tenosynovitis (BT), which involves the biceps brachii muscle and its tendon that crosses the shoulder joint (Figure 33-2). The biceps flexes the elbow and extends and stabilizes the shoulder joint during standing or during the weight-bearing phase of locomotion.22 Repeated strain injury appears to be the primary cause of BT in the performance dog. This includes two-on/two-off contacts, landing vertically on the forelimbs from a misjudged jump, overstretching of the muscle, quick turns, and repetitive contractions of the muscle with the shoulder flexed and/or the elbow extended. Injury to the tendon can occur in a number of ways, including strain from overloading, degeneration, or disruption.23 A single less than maximum load may injure some of the fibers without complete failure of the tendon, but the blood supply to the tendon proper is poor, leading to a longer healing time.21 Repetitive strain injury may initiate actual degeneration of the tendon.21 As the area continues to be reinjured, the tendon may weaken sufficiently for inflammation or microtears (tendinopathies) in the connective tissue in or around other tendons to form, ultimately leading to shoulder joint instability.24 Performance dogs with BT commonly have difficulty with quick turning to the affected side and are reluctant to jump. Agility dogs may also have issues performing two-on/two-off contacts and knocking bars with their forelimbs. On gait analysis of dogs with BT often have a shortened stride and a weight-bearing lameness (subtle to severe) that becomes worse with activity. Pain may be elicited by direct palpation over the biceps tendon. Pain and spasm may be noted when flexing the shoulder while at the same time extending the elbow (Figure 33-3).3 Radiographs are of minimal assistance when the injury is in its acute phase, but may reveal mineralization of the tendon when the condition is chronic.24 Magnetic resonance imaging (MRI) and ultrasound may be used to identify the condition in acute and chronic situations.23,24 Shoulder arthroscopy is recognized as superior to other diagnostic procedures for diagnosis of bicipital tenosynovitis (Figures 33-4 and 33-5).23 Acute cases of bicipital tenosynovitis may be treated by conservative medical management and rehabilitation therapy. Components of conservative medical management should include controlled activity, nonsteroidal antiinflammatory drugs (NSAIDs), and cryotherapy. Intraarticular injections of hyaluronic acid or steroids, or intra-tendinous injections of platelet rich plasma (PRP), stem cell therapy (SCT) or a combination of these may be considered.20,23–25 Regenerative medicine therapy should be considered if a core lesion is present. To promote healing and decrease adhesion formation, laser therapy or pulsed ultrasound may be of use to assist healing to (Figure 33-6).20 Initiating low-load, pain-free, high-repetition exercise and range of motion (ROM) movements early in the healing process is important. Pain relief may be achieved with transcutaneous electrical nerve stimulation.20 Acute tendon injuries should not be stretched because of the potential for producing more microtears caused by the chemical changes in the tissue.1,20 Pain-free, passive ROM movements (flexion and extension of the limb while the dog is not weight bearing) and gentle wobble board activities (all directions, front to back and side to side), and alternating leg lifts are recommended first, with progression to active ROM exercises (weight-bearing exercises that cause flexion and extension of the limb). All exercises in the acute stage are aimed at increasing the strength of the shoulder and scapular stabilizers. Balance and proprioceptive exercises are required and are imperative to the healing process. An active dog requires stabilization of this region and strength of the slow twitch muscle fibers before resuming activity. Deep friction massage is used at this stage if it is not too painful.25,26 Deep friction massage involves a sweeping motion made across the bicipital tendon (perpendicular to the fibers) for 5 minutes, daily if possible. The goal is to prevent adhesion formation and help realign the fibers of the inflamed tendon. After the cross-friction massage, cryotherapy and laser therapy are recommended before further treatment. A strengthening program consisting of controlled leash walking, stepping over cavaletti rails, wobble board use, and stabilizing exercises should follow. Examples of stabilization exercises include balancing on a well-controlled rocker board, standing on a therapy ball, weight shifting exercises, wheel-barrowing the dog so its weight is on the forelimbs, walking in figure eights, and walking in circles. Leash walks of increasing duration and end-stage eccentric exercises, such as walking and trotting down hills may then be introduced. Short periods of off-lead activity are allowed after leash walks have substantially increased without the return of lameness. In the acute stages, it is imperative to reinforce the owner or handler not to perform any activity that produces lameness. Many owners of performance dogs are eager to return to sports and often push their dogs too quickly. Surgical treatment is recommended for biceps avulsion-tears and chronic cases of BT that are not responsive to medical management or rehabilitation therapy. Surgical options include tenodesis (releasing and reattaching the tendon at a distal location on the proximal humerus) or an arthroscopic tendon release.27 Controlled activity is an important component during the postoperative period and should include leash walks of increasing duration and end-stage eccentric exercises, such as walking and trotting down hills. Short periods of off-lead activity are allowed after the lameness has resolved. Nonsteroidal antiinflammatory drugs may be used to decrease pain and inflammation following surgery. Modalities as described for the acute treatment of BT may be used postoperatively to improve comfort and function and facilitate progression to the retraining phase of recovery. Dogs that will be returning to jumping activities should start with low-level activities before returning to the sport.4,26 In jumping activities, the biceps muscle and tendon act eccentrically to control the landing of the body. After cavaletti work has been employed, low-level jumping can be initiated. The jump heights should be set lower than the dog’s normal jump height. For example, if the dog normally jumps 22 inches in competition, the jump height should be set at 16 inches. Three or four jumps should be set up and the surface should be padded or the exercise should be performed in sand. Three to five repetitions should be performed of the jump sequence—the dog going over the jumps—and then the dog should be reevaluated. The jump sequencing should not cause any lameness and should only be performed three times per week with at least a 48-hour break in between. Plyometrics should be progressed by increasing the jump height and the number of repetitions. Once the dog is up to its normal jump height on a controlled surface, additional training surfaces can be added. In humans and dogs several degenerative disorders of the supraspinatus tendon have been identified, including rotator cuff tears, calcifying tendinitis or tendinosis (microtears), and tendinosis as a result of overuse.28,29 In humans degeneration of the supraspinatus tendon is believed to be a contributing factor in the development of rotator cuff tears.29 There is good evidence that overuse is likely an important factor in this disorder. At the cellular level affected tendons contain discontinuous, disorganized fibers, and typically, no acute inflammation is detected.29 In chronic cases a rapidly growing nodule develops that can impinge on the biceps brachii tendon and cause pain.30 The supraspinatus muscle extends the shoulder and advances the limb. The muscle is important to stabilize and prevent collapse of the shoulder joint and is active during 65% to 80% of the time when the dog is standing (Figure 33-7).22 Performance dogs with supraspinatous tendinopathy (ST) commonly have weight-bearing lameness on one side that becomes worse with activity and often is resistant to treatment. The supraspinatus muscle may be atrophied and direct palpation over the tendon and flexion of the shoulder may cause pain (Figure 33-8). Concurrent BT or shoulder instability may be present, so a thorough shoulder examination is required. Magnetic resonance imaging readily demonstrates the condition in the acute phase (Figure 33-9). Plain radiographs and computed axial tomography (CT), which reveals a cross-section of an internal body structure) may reveal mineralization in chronic cases. Musculoskeletal ultrasound is this author’s preferred diagnostic tool for this condition. This technique provides a non-invasive definitive diagnosis of ST and allows for serial evaluations to assess response to treatment. Arthroscopic exploration is recommended and may demonstrate impingement of the biceps tendon secondary to supraspinatus tendon swelling as well as concurrent pathologies, including medial shoulder instability in severe cases. Controlled activity, retraining, and preventive techniques for acute and chronic ST is similar to that used for bicipital tenosynovitis. Similar to the rehabilitation of BT, rehabilitation should be initially aimed at reducing the inflammation and increasing the strength of the shoulder and scapular stabilizers.4 After the inflammation has decreased and the core strength of the shoulder complex has improved, more dynamic activities may be initiated. Treatment for acute cases consists of conservative medical management (controlled activity and NSAIDs) and rehabilitation therapy (manual therapy, modalities, ROM with progression to stretching and strengthening exercises), and acupuncture as described for BT.20 Cross-friction massage may be performed to the supraspinatus tendon in the acute phases for 5 minutes daily and 10 to 15 minutes in chronic cases. As with BT the inflammatory process should be reinitiated in chronic cases and follow a similar rehabilitation protocol. Shockwave and ultrasound therapy have also been described as valuable modalities for the treatment of chronic calcified supraspinatus tendinopathies.31 Surgical treatment is warranted for chronic cases that do not respond to conservative medical management and rehabilitation therapy. Surgical treatment should include arthroscopic exploration to identify and treat possible concurrent BT or shoulder instability, or removal of abnormal tissue through an open approach.30 Surgery has been considered the treatment of last resort in humans with tendinosis because of the small reported difference in treatment success between surgical intervention and conservative management. Rehabilitation therapy, as described for BT, following surgical intervention is essential in returning the canine athlete to performance. Contracture of the infraspinatus muscle is a rare condition most often affecting large breed dogs, particularly working dogs (i.e., hunting dogs, herding dogs) and other very active dogs.32 Infraspinatus muscle contracture was first reported in the Dutch literature in 197033 and is one of the two most frequently reported contractures in the dog, along with quadriceps contracture.34,35 Unilateral contraction of the infraspinatus muscle is most common; however, bilateral contractures have also been reported.33,36,37 Most cases are working or hunting dogs that present with an acute onset of shoulder lameness with work or exercise.33,34,36–40 Causes reported include repetitive microtrauma, blunt trauma, and osteofascial compartment syndrome.41,42 Compartment syndrome is a disease process seen in people; however, it can also occur in dogs and horses.43 The pathology to muscle tissue is caused by an increase in pressure in a confined, inelastic space that leads to muscle inflammation with no room to expand.43 Muscle necrosis occurs when the pressure rises to greater than 30 mm Hg (normal, −2 to 8 mm Hg) in a single compartment with no relief.43 A compartmental pressure greater than 120 mm Hg may lead to complete and irreversible nerve block.44 Causes for an increased pressure within a compartment include hemorrhage, postischemic tissue swelling, external pressure (bandage), or intramuscular injection.32 In elastic barriers that predispose to compartment syndrome there are structures such as fascia and bone.43 Four different compartments have been described in the dog: femoral, craniolateral crus, caudal crus, and caudal antebrachial.43,44 It may be possible that compartment syndrome may play a role in the contracture of the muscles of the shoulder joint. If an injury is sustained to the infraspinatus muscle that leads to moderate cellular inflammation, the muscle begins to swell and has no room to expand. Inflammation within the boundaries of the deltoids, scapula, and surrounding fascia increase the compartmental pressure and inhibit venous blood flow, initially causing congestion. Then arterial blood flow and innervation are affected, as well as diminished cellular response to damaged tissue. It is the unforgiving pressure that leads to further cellular damage. Cellular swelling caused by pressures rising to greater than 30 mm Hg is what seems to lead to necrosis and/or fibrosis of the affected muscles. Early treatment is focused on surgical decompression by fasciotomy; however, if contraction has already occurred, tenectomy or myectomy may be necessary.45 Acutely the shoulder is swollen and very painful. The initial trauma usually resolves with rest and supportive treatment. However, the affected limb begins to show signs of contracture several days to weeks after the initial trauma.40 Physical examination of a chronic case usually indicates a bright, alert dog, without signs of any systemic illness. There is usually a weight-bearing lameness of the affected forelimb and a characteristic gait. Palpation and manipulation of the contracted limb does not usually elicit a pain response.38,40 The remaining shoulder muscles, mainly the supraspinatus40 and deltoid muscles, may show signs of disuse atrophy or be contracted as well.33,34,39,40 A substantial decrease in range of motion of the shoulder joint is appreciated.40 The affected forelimb is usually held with the shoulder in abduction and the elbow in adduction against the thoracic wall38 (Figure 33-10). The distal forelimb and carpus are externally rotated and held in abduction.36,40 Gait abnormalities usually consist of weight-bearing lameness and circumduction33,38–40 of the affected forelimb with a characteristic fliplike action of the paw when placing the foot.36,40 When the patient is placed in lateral recumbency with the affected limb up, a true infraspinatus contracture will cause the distal forelimb to remain in an abducted and externally rotated position off the floor and away from the body. Siems et al. reported a series of ultrasonographic findings of two concurrently traumatized infraspinatus muscles in the same dog; one resolved without complications, and the other resulted in contracture.39 The primary ultrasonographic description of a contracted infraspinatus muscle is increased echogenicity when compared with a normal supraspinatus muscle.36,39 Magnetic resonance imaging is another diagnostic modality that may be useful for diagnosing the condition in both the acute and chronic phases. Physical rehabilitation of acute or subacute infraspinatus tendinopathies may be used to maintain joint motion and prevent contracture. Continuous ultrasound and stretching exercises may help maintain the length and mobility of the affected tissues. Mature contractures most likely require surgical release. Mature contractures are treated with a tenotomy of the tendon of insertion of the infraspinatus muscle and release of capsular adhesions of the tendon. Infraspinatus muscle contracture carries an excellent prognosis with tenectomy of the infraspinatus tendon.40,46 Lateral or craniolateral approaches to the shoulder both provide good exposure of the infraspinatus tendon.33,40,41 Complete transection of the infraspinatus tendon with removal of a length of the tendon is required to restore motion in the affected limb and most often proves curative with an immediate release of contracture and return to function of the shoulder joint.36,40 It is common to find adhesions to the infraspinatus tendon and joint capsule during surgery; however, after they are dissected away they seldom cause further problems.40,41 Postoperative bandages need not be applied, and rehabilitation therapy should start when sutures are removed to prevent the formation of further adhesions.40 Medial shoulder syndrome (MSS) is one of the most common causes of forelimb gait related issues and lameness in performance dogs. This condition may be considered somewhat similar to rotator cuff injuries in people (including ligament disruption; tendinopathy; labral and capsular tears or disruption). In human athletes, an overhead throwing motion involves intricate motion combined with proper stabilization. During this activity, the shoulder must be lax enough to allow extremes of motion but secure enough to provide stabilization.4,26 The same is true for the canine athlete. Many routine maneuvers performed by dogs also involve extremes of ROM and require appropriate stabilization. In dogs with medial shoulder instability (MSI), lameness may be as subtle as performance-related problems such as refusing tight turns or as severe as a weight-bearing lameness. MSI involves multiple components of the shoulder joint. Definitive diagnosis requires a thorough orthopedic evaluation and advanced diagnostic tests. Medial shoulder instability is diagnosed when significant shoulder laxity is present. Early identification and treatment offer the best long-term result and prevent further shoulder instability and progression of osteoarthritis. Anatomically, the shoulder joint is one of the least stable joints in the dog, relying on soft tissue structures for stability and function (Figure 33-11).46 In dogs the glenohumeral (shoulder) joint has the widest ROM and is capable of movements in any direction, but its primary movements during ambulation are flexion and extension.46 The glenohumeral joint relies heavily on capsuloligamentous support to provide stability because its anatomic conformation does not provide a stable socket.46 The humeral head and glenoid cavity of the scapula make up the ball and socket of the shoulder joint. The glenoid provides little coverage of the humeral head. The joint capsule, ligaments, and surrounding muscles and tendons all contribute to the stability of the shoulder. Insult or injury to any portion of the capsuloligamentous support structures can potentially cause shoulder joint pathology resulting in pain and dysfunction.47 The components that are most commonly affected by MSS and MSI include the craniomedial joint capsule, medial glenohumeral ligament, subscapularis tendon, supraspinatus tendon, labrum, and less commonly the biceps tendon (shown in Figures 33-12 and 33-13). Depending on the severity and chronicity of the condition, the cartilage may also be affected. Currently, the exact cause of MSS in dogs is unknown, although it is suspected to be related to chronic repetitive activity or overuse rather than trauma. Overuse of the shoulder support structures leads to degeneration of the tissues, lowering the tensile strength of the tissues and predisposing them to fraying, disruption, and eventually complete breakdown.1,20 Sporting athletes that participate in activities such as agility undergo extreme stresses on their muscles, ligaments, and tendons. Repetitive activities such as jump-turn combinations and weave poles performed regularly during practice and at weekend trials place the shoulder near its end range of abduction (as shown in Figure 33-14), and stress the soft tissues of the medial shoulder complex. Additionally, events such as slipping on wet surfaces, mishaps on the dog walk, teeter, or A-frame may also contribute to the trauma inflicted on the shoulder while participating in agility. Over time there may be a cumulative effect of the microtrauma occurring to the ligaments, tendons, and joint capsule, leading to a decrease in performance. Because preoperative diagnosis of MSS is critical for clinical decision making, a palpation technique has been developed known as the shoulder abduction test.48 Although this test was originally described in dogs under sedation in lateral recumbency, it is noted by the author that this test may be performed with similar consistent results on dogs awake and standing. To appropriately engage the components of the craniomedial shoulder, the elbow and shoulder must be placed in full extension with concurrent abduction of the forelimb. The scapula at the level of the acromion process must be stabilized with the evaluator’s opposite hand to achieve a passive stretch of the craniomedial shoulder components. A goniometer is held such that one arm of the goniometer runs parallel to the spine of the scapula so that the center of rotation of the goniometer is centered over the shoulder joint and the free arm of the goniometer extends over the axis of the humerus in contact with the skin. With the elbow and shoulder held in extension, the forelimb is abducted to its physiologic limit and the measurement obtained as shown in Figure 33-15. In severe cases, which clinically appear to be much less common, a slight “thud” or subluxation may be felt when abducting the shoulder. The procedure should then be performed on the contralateral shoulder for comparison. Using this palpation technique, it has been reported that a normal mean shoulder abduction angle is approximately 30 degrees.48 Although this report revealed that dogs with medial shoulder instability had abduction angles significantly larger than 30 degrees,48 a more recent study showed an inconsistent correlation between abduction angles and medial compartment pathology identified arthroscopically.49 This is also the author’s clinical observation. Dogs with MSS rarely show evidence of “instability,” but rather show signs of dysfunction and pain associated with craniomedial pathology, including varying degrees of subscapular tendinopathy; medial glenohumeral ligament (MGL) disruption; craniomedial capsular lesions; and labral tears. Because MSS is usually a unilateral problem, the contralateral shoulder usually does not show signs of dysfunction, pain, or spasm on abduction.
Common Conditions and Physical Rehabilitation of the Athletic Patient

Muscle Strains
Regenerative Medicine Therapy for Musculoskeletal Injury
Forelimb Conditions
Bicipital Tenosynovitis
Cause
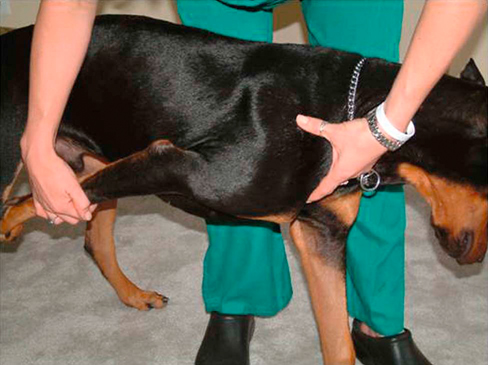
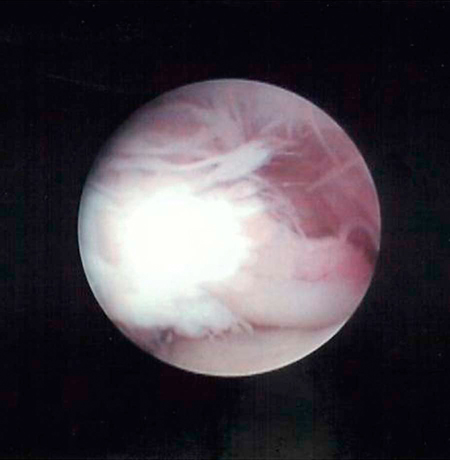
Diagnosis
Treatment
Supraspinatus Tendinopathy
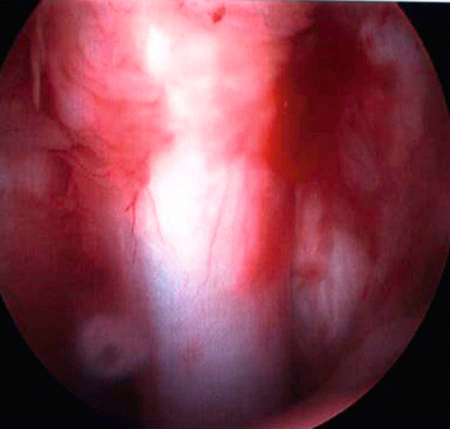
Diagnosis
Treatment
Infraspinatus Contracture
Cause
Diagnosis
Treatment
Medial Shoulder Syndrome and Medial Shoulder Instability
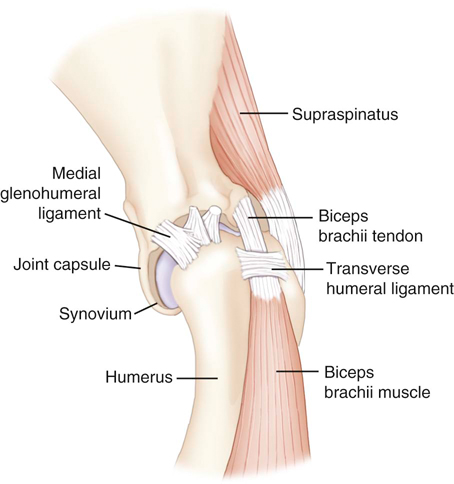
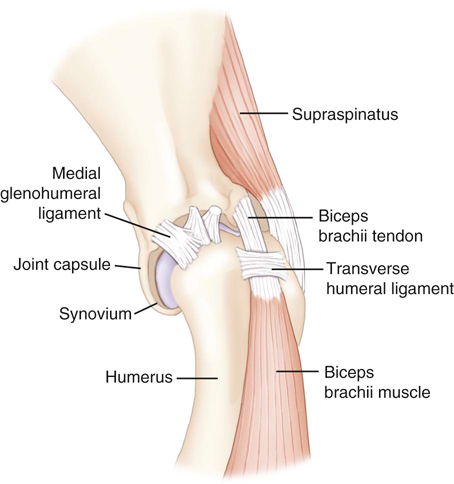
Anatomy
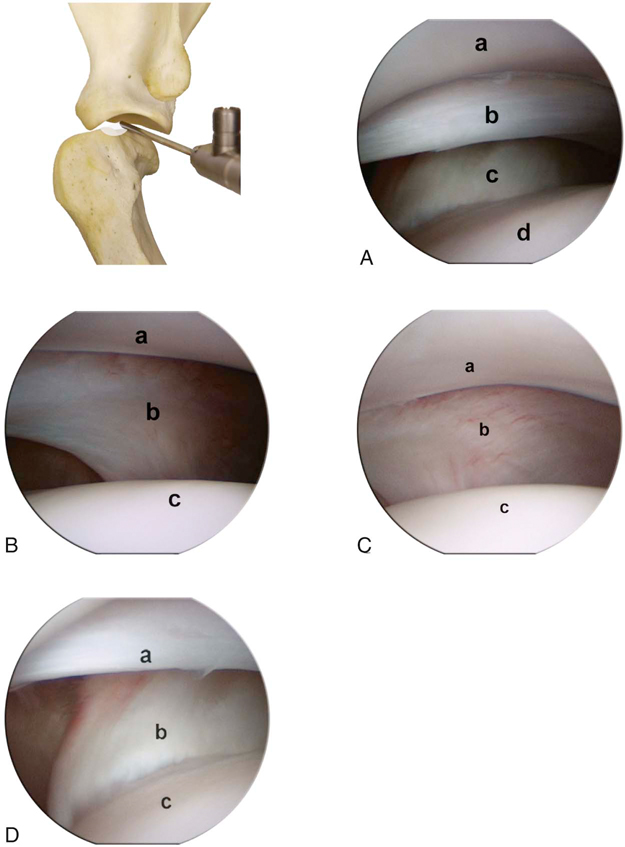
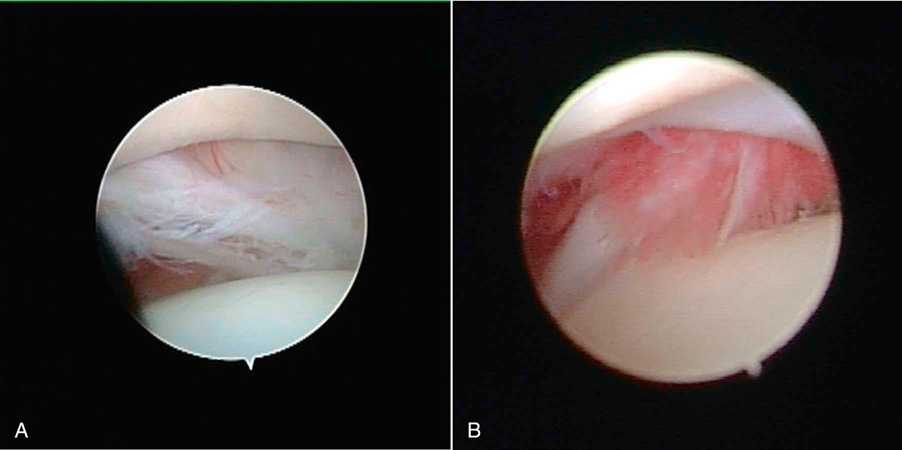
Figure 33-12 A, Arthroscopic view of the craniomedial compartment of the shoulder joint. Glenoid (a), cranial arm of the medial collateral ligament (MCL) (b), subscapularis tendon (c), and humeral head (d). B, Arthroscopic view of the centromedial compartment of the shoulder joint. Glenoid (a), centromedial area of the MCL (b), and humeral head (c). C, Arthroscopic view of the caudomedial compartment of the shoulder joint. Glenoid (a), caudomedial area of the MCL (b), and humeral head (c). D, Arthroscopic view of the cranial compartment of the shoulder joint. Cranial arm of the MCL (a), insertion of the subscapularis tendon (b), and humeral head (c). (From Beale, B.; Hulse, D.; Schulz, K.; Whitney, W.: Small Animal Arthroscopy, 2003, Saunders.)
Cause
Diagnosis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Common Conditions and Physical Rehabilitation of the Athletic Patient
Only gold members can continue reading. Log In or Register to continue