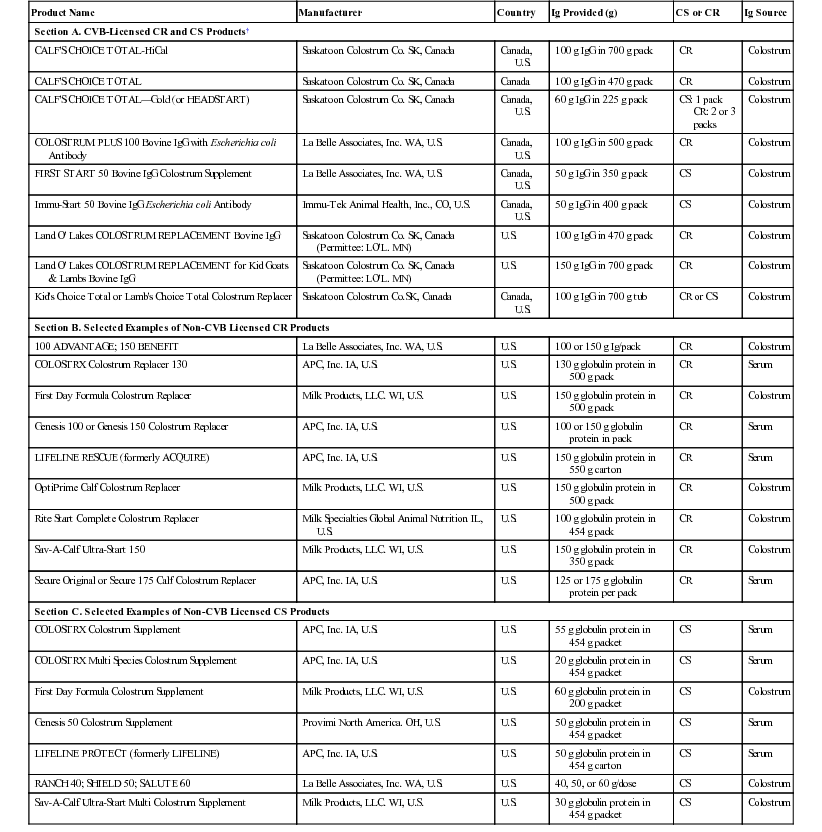
Sandra Godden, Robert E. James, Consulting Editors The goals of colostrum and milk feeding programs for preweaned calves and small ruminants are to achieve optimal rates of gain, support and develop a strong immune system, maximize health, and stimulate ruminal development—all in a cost-effective manner. Although feeding high-quality, clean maternal colostrum and whole milk may be considered by many to be gold standards, the use of high-quality colostrum replacement products and milk replacers may be attractive to producers for a variety of reasons, including for consistency, for convenience, as a means of breaking the transmission cycle of some infectious diseases, or simply to capture economic efficiencies. This chapter reviews important considerations in the manufacture, selection, and use of high-quality colostrum replacement and milk replacer products. The principles of colostrum management are reviewed in Chapter 19. Although feeding 3 to 4 L of clean, high-quality maternal colostrum shortly after birth remains the gold standard, farms can experience periods when an adequate supply of high-quality fresh or stored maternal colostrum is not available. Also, it may not always be practical or convenient to harvest first milking colostrum from fresh cows within a timely manner after calving (<1 to 2 hours). Contributing to this shortage, some producers may discard colostrum from cows testing positive to such pathogens as Mycobacterium avium subsp. paratuberculosis or Mycoplasma bovis.1–3 Under such circumstances, feeding commercially available colostrum supplement (CS) or colostrum replacement (CR) products may offer a simple, consistent and convenient means of delivering the necessary immunoglobulins (Ig) to newborn calves while reducing the risk of pathogen exposure. The following section discusses types of CS and CR products, manufacture and licensing, evaluation of product efficacy (e.g., IgG dose, efficiency of IgG absorption, passive transfer levels, calf health and performance), impact of Ig source on antibody profiles, monitoring of passive transfer, and use of CR or CS products in minor species. Although some studies have demonstrated that supplementation of the milk diet with Ig from either maternal colostrum or CS may reduce the severity of rotavirus or coronavirus scours occurring during the first 14 days, the current discussion is limited to use of CS and CR products in the first 24 hours after birth.4–6 Colostrum supplements, which typically cost between $9 and $18 USD per dose, are designed to provide exogenous IgG to calves to supplement poor-quality maternal colostrum (colostral IgG < 50 g/L).7 Most CSs provide between 25 and 60 g of IgG per dose, which is reconstituted in 1 to 2 L of water. Feeding one dose of a CS will not provide a sufficient mass of IgG to prevent failure of passive transfer (FPT) in young calves, nor will it provide sufficient levels of nutrients necessary for calf survival or optimum growth.7,8 By comparison, CR products, which typically cost between $25 and $40 USD per dose, are designed to be fed instead of maternal colostrum. They should provide a minimum of 100 g of IgG per dose and should provide sufficient levels of nutrients to the calf. It is recommended that CS or CR products be mixed in water, according to the manufacturer’s label directions, and fed as a separate meal after any natural colostrum has been fed.9 Immunoglobulins in commercial CR and CS products are most often derived from spray-dried bovine colostrum or spray-dried bovine serum. For colostrum-derived CR products, fresh-frozen bovine colostrum is collected from grade A dairies and assembled at a manufacturing plant where it undergoes testing for IgG concentration, pooling, heat treatment, spray drying, and then packaging. The concentrations of most non-Ig colostral components (e.g., nutrients, hormones, growth factors), with the exception of cellular fractions (i.e., leukocytes, bacteria), will be similar in colostrum-derived CR products compared with maternal colostrum. For serum-derived CR or CS products, blood is collected from abattoirs under USDA inspection, centrifuged to separate plasma and/or serum from red blood cells, ultrafiltered, spray dried to produce a concentrated powder containing approximately 20% Ig, and then mixed with other ingredients (e.g., lactose, dry fat blend, dextrose, vitamin/mineral premix). The final product may be irradiated. Exact manufacturing methods will vary among manufacturers and products. In Canada, it is required that all CR products are licensed through the Canadian Food Inspection Agency (CFIA) Center for Veterinary Biologics (CVB, Ottawa, Ontario, Canada).10 Serum- or plasma-derived CR or CS products are not permitted in Canada. In the United States, only some manufacturers have acquired a license through the U.S. Department of Agriculture (USDA) Center for Veterinary Biological Products (CVB, Ames, IA). To attain a CVB license and label for prevention or treatment of FPT of Ig, the IgG in U.S. CR products must come from bovine colostrum collected from grade A dairies, must be processed by heat treatment or ionizing radiation using accepted protocols, and must undergo regular purity, potency, and efficacy testing.11,12 Purity requirements allow a specified total bacteria count. However, coliforms, Salmonella spp., and fungi may not be present. Potency tests require a minimum IgG content for FPT products as determined by a validated radial immunodiffusion (RID) kit or assay. Efficacy testing for FPT products requires that, in 90% of animals, the increase in IgG concentration (pretreatment vs. 24-hour posttreatment) must be at least equal to concentration of the IgG Species Standard approved by the USDA’s Animal and Plant Health Inspection Service (APHIS) (e.g., ≥10 mg/mL serum IgG for neonatal calves) (USDA: 9CFR 113.450; 9CFR 113.499).11 Samples of product from every serial release (batch) are submitted to a central CFIA or USDA laboratory for testing. Also, the overseeing regulatory body completes annual site inspections to review processes and records. As such, licensed products can guarantee safety, potency, efficacy, and traceability. Table 21-1 (Section A) lists CVB-licensed products currently available in Canada and the United States. TABLE 21-1 CVB-Licensed and Selected Non-Licensed Colostrum Replacements and Supplements* * Product list may change in the future; specifications may change in the future, so read the label. CR, Colostrum replacement; CS, colostrum supplement; CVB, Centers for Veterinary Biologics (CFIA or USDA). Although products that do not have a USDA Veterinary Biologics license are not legally able to claim to supply IgG or to purport to be used as either CS or CR for prevention of FPT, their use for this purpose is widespread in the United States. The Ig in nonlicensed products is technically not considered a feed but is being used in feeds. As such, these products fall under Association of American Feed Control Officials (AAFCO) guidelines (www.aafco.org). Under AAFCO guidelines, every state (i.e., local State Department of Agriculture) adopts and regulates its own animal feed per its applicable laws and regulations. Feed testing programs are in place and regulated in Canada. In the United States, there is a system to identify feed products, but there currently is no system to regulate them through the USDA, FDA, or the State Departments of Agriculture. Although all U.S. manufacturers of non-CVB licensed CR and CS products will conduct some kind of internal quality control testing, these programs are at the manufacturer’s discretion. External product testing or plant inspections by regulatory authorities (e.g., State Department of Agriculture) are not typically completed for non-CVB licensed products unless a problem or complaint is brought to their attention. Table 21-1 (Sections B and C) lists some of the many non-CVB licensed CR and CS products available in the United States. Producers are strongly encouraged to select CR or CS products that have undergone independent evaluation of efficacy in controlled field studies. Evaluating efficacy should ideally consider passive transfer of adequate IgG, nutritional support, and calf health and performance. Passive transfer of IgG will be primarily determined by the dose of IgG (in grams) administered as well as the efficiency of absorption of IgG. Each product must be independently evaluated, since differences in manufacturing processes can have a significant impact on both apparent efficiency of absorption percentage (AEA %) and final dose of IgG in a package.13,14 Head-to-head studies are necessary to make valid comparisons among products. Table 21-2 presents the results of field efficacy studies for commercially available CR and CS products that were mixed and fed according to label directions. TABLE 21-2 Selected Efficacy Studies of CS and CR Products* * Limited to studies of commercially available products mixed and fed according to label directions. ¶ Product licensed through Center for Veterinary Biologics (CFIA and/or USDA). †,‡,§,||Means within column within study with different superscripts differ (P < 0.05). AEA, Apparent efficiency of absorption of IgG (%); APT, proportion (%) of calves with acceptable passive transfer (serum IgG ≥ 10 mg/mL); CD, colostrum-derived IgG source; CR, colostrum replacement; CS, colostrum supplement; N, number of calves per treatment group; NR, not reported; SD, serum-derived Ig source. Although experts originally estimated that producers should feed a minimum mass of 100 g of IgG in the first colostrum feeding, more recent studies have shown that at least 150 to 200 g of colostral IgG must be fed to consistently achieve acceptable passive transfer (APT) rates (serum IgG ≥ 10 mg/mL ) in the majority (≥90%) of calves.15–18 This dose effect was demonstrated in one study wherein three groups of calves fed either 100 or 200 g of IgG in a colostrum-derived CR product or 3.8 L of fresh maternal colostrum achieved a mean serum IgG concentration at 24 hours of 9.6 mg/mL (46% with FPT), 19.0 mg/mL (0% with FPT), or 20.7 mg/mL (9% with FPT), respectively (see Table 21-2).18 Although many CR products are packaged to provide 100 to 130 g IgG per dose, some CR products now provide a larger mass of IgG per dose and/or provide label directions that suggest feeding increased masses of IgG. Grams are more important than percentages; under most circumstances, the total dose (g) of IgG fed will be the most important predictor of APT in calves fed CR products. That said, simply examining the mass of IgG provided by the CR is not an adequate predictor of product efficacy.19 There can be significant differences in AEA % among products resulting from, but not limited to, such factors as manufacturing methods and quantity, the type of other nutrients or additives, and the age (in hours) at which the calves were fed. The apparent efficiency of absorption (AEA %) is the percentage of IgG administered that is absorbed systemically.13 To calculate AEA %, one needs to know the serum IgG (mg/mL is equal to g/L), the grams of IgG originally fed (estimated from testing the maternal colostrum or indicated on the label of the CR or CS fed), and the weight of the calf (in kilograms) from which the plasma volume is estimated (e.g., plasma volume in liters = 9.1% of body weight in kg). The serum IgG (g/L) is multiplied by the plasma volume (L) to calculate the total grams of IgG in the blood. This is then divided by the total grams of IgG fed, and then multiplied by 100 to express efficiency as a percentage. For example, a 43-kg calf has an estimated 3.9 L of plasma (43 kg × 0.091). If the serum IgG concentration of the calf is 10 g/L (10 mg/mL), then the calf has 39 g of IgG in the blood (10 g/L × 3.9 L). If that calf was originally fed 100 g of IgG from maternal colostrum or a CR product, then the AEA would be 39% (39 g/100 g × 100%). Controlled field studies using commercially available CR or CS products, mixed and fed according to label directions, have reported widely varying AEA % values (see Table 21-2). The AEA % reported for CS products has ranged from 16% to 28%.20,21 The AEA % reported for CR products has ranged from 14% to 51%.18,22–29 By way of comparison, the AEA % of IgG reported for calves fed 3 to 4 L of fresh maternal colostrum within 6 hours of birth has ranged from 21% to 36%.18,20,21,27,29 The debate continues over whether the IgG in colostrum- or serum-based CR or CS products is more efficiently absorbed. However, relatively few head-to-head field trials exist to investigate this question. In one early CS study in which calves were fed two doses of a colostrum-derived CS (50 g IgG), two doses of either 60 g or 90 g IgG of serum-derived CS, or 3.8 L of maternal colostrum, the AEA % reported for the colostrum-derived CS (16%) was inferior to that of the two serum-derived CS products (24% or 28%) and to maternal colostrum (22%) (see Table 21-2).20 However, because all three CS products delivered an insufficient dose of IgG as compared with maternal colostrum, all three CS products failed to provide APT to calves. Thus far, only two head-to-head controlled field studies have compared the AEA % of commercially available serum- versus colostrum-derived CR products. In one study calves fed 150 g of a lacteal-derived CR had significantly improved AEA % and serum IgG concentrations (AEA = 38.2%; IgG = 14.7 mg/mL) as compared with calves fed 130 g of a serum-derived CR (AEA = 28.4%; IgG = 9.6 mg/mL) (see Table 21-2).26 In this study the improved final serum IgG values for the colostrum-derived CR group were attributed both to increased dose fed (in grams) and improved AEA %. In another recent study, calves fed 150 g of a serum-derived CR had inferior AEA % (21.6%) compared with calves fed 100 g of a colostrum-derived CR (38.8%).30
Colostrum and Milk Replacers

Colostrum Supplements and Replacements
Colostrum Supplements versus Colostrum Replacements
Manufacturing
Is the Colostrum Replacer or Colostrum Supplement Licensed?
Product Name
Manufacturer
Country
Ig Provided (g)
CS or CR
Ig Source
Section A. CVB-Licensed CR and CS Products†
CALF’S CHOICE TOTAL-HiCal
Saskatoon Colostrum Co. SK, Canada
Canada, U.S.
100 g IgG in 700 g pack
CR
Colostrum
CALF’S CHOICE TOTAL
Saskatoon Colostrum Co. SK, Canada
Canada
100 g IgG in 470 g pack
CR
Colostrum
CALF’S CHOICE TOTAL—Gold (or HEADSTART)
Saskatoon Colostrum Co. SK, Canada
Canada, U.S.
60 g IgG in 225 g pack
CS: 1 pack
CR: 2 or 3 packs
Colostrum
COLOSTRUM PLUS 100 Bovine IgG with Escherichia coli Antibody
La Belle Associates, Inc. WA, U.S.
Canada, U.S.
100 g IgG in 500 g pack
CR
Colostrum
FIRST START 50 Bovine IgG Colostrum Supplement
La Belle Associates, Inc. WA, U.S.
Canada, U.S.
50 g IgG in 350 g pack
CS
Colostrum
Immu-Start 50 Bovine IgG Escherichia coli Antibody
Immu-Tek Animal Health, Inc., CO, U.S.
Canada, U.S.
50 g IgG in 400 g pack
CS
Colostrum
Land O’ Lakes COLOSTRUM REPLACEMENT Bovine IgG
Saskatoon Colostrum Co. SK, Canada (Permittee: LO’L. MN)
U.S.
100 g IgG in 470 g pack
CR
Colostrum
Land O’ Lakes COLOSTRUM REPLACEMENT for Kid Goats & Lambs Bovine IgG
Saskatoon Colostrum Co. SK, Canada (Permittee: LO’L. MN)
U.S.
150 g IgG in 700 g pack
CR
Colostrum
Kid’s Choice Total or Lamb’s Choice Total Colostrum Replacer
Saskatoon Colostrum Co.SK, Canada
Canada, U.S.
100 g IgG in 700 g tub
CR or CS
Colostrum
Section B. Selected Examples of Non-CVB Licensed CR Products
100 ADVANTAGE; 150 BENEFIT
La Belle Associates, Inc. WA, U.S.
U.S.
100 or 150 g Ig/pack
CR
Colostrum
COLOSTRX Colostrum Replacer 130
APC, Inc. IA, U.S.
U.S.
130 g globulin protein in 500 g pack
CR
Serum
First Day Formula Colostrum Replacer
Milk Products, LLC. WI, U.S.
U.S.
150 g globulin protein in 500 g pack
CR
Colostrum
Genesis 100 or Genesis 150 Colostrum Replacer
APC, Inc. IA, U.S.
U.S.
100 or 150 g globulin protein in pack
CR
Serum
LIFELINE RESCUE (formerly ACQUIRE)
APC, Inc. IA, U.S.
U.S.
150 g globulin protein in 550 g carton
CR
Serum
OptiPrime Calf Colostrum Replacer
Milk Products, LLC. WI, U.S.
U.S.
150 g globulin protein in 500 g pack
CR
Colostrum
Rite Start Complete Colostrum Replacer
Milk Specialties Global Animal Nutrition IL, U.S.
U.S.
100 g globulin protein in 454 g pack
CR
Colostrum
Sav-A-Calf Ultra-Start 150
Milk Products, LLC. WI, U.S.
U.S.
150 g globulin protein in 350 g pack
CR
Colostrum
Secure Original or Secure 175 Calf Colostrum Replacer
APC, Inc. IA, U.S.
U.S.
125 or 175 g globulin protein per pack
CR
Serum
Section C. Selected Examples of Non-CVB Licensed CS Products
COLOSTRX Colostrum Supplement
APC, Inc. IA, U.S.
U.S.
55 g globulin protein in 454 g packet
CS
Serum
COLOSTRX Multi Species Colostrum Supplement
APC, Inc. IA, U.S.
U.S.
20 g globulin protein in 454 g packet
CS
Serum
First Day Formula Colostrum Supplement
Milk Products, LLC. WI, U.S.
U.S.
60 g globulin protein in 200 g packet
CS
Colostrum
Genesis 50 Colostrum Supplement
Provimi North America. OH, U.S.
U.S.
50 g globulin protein in 454 g packet
CS
Serum
LIFELINE PROTECT (formerly LIFELINE)
APC, Inc. IA, U.S.
U.S.
50 g globulin protein in 454 g carton
CS
Serum
RANCH 40; SHIELD 50; SALUTE 60
La Belle Associates, Inc. WA, U.S.
U.S.
40, 50, or 60 g/dose
CS
Colostrum
Sav-A-Calf Ultra-Start Multi Colostrum Supplement
Milk Products, LLC. WI, U.S.
U.S.
30 g globulin protein in 454 g packet
CS
Colostrum
Evaluating the Efficacy of Colostrum Replacement Products
Citation
Colostrum Supplement or Replacer Product Fed
N
Total IgG Fed (g)
Age Fed (hr)
AEA IgG (%)
Serum IgG (mg/mL)
APT (%)
Colostrum Supplement Studies
Arthington et al, 200020
First Milk Formula CS (CD)
10
50 g (2 doses)
<2 h & 12 h
≈16%†
2.2†
NR
Colostrx CS (SD)
10
60 g (2 doses)
<2 h & 12 h
≈24%‡,§
3.5‡
Lifeline CS (SD)
10
90 g (2 doses)
<2 h & 12 h
≈28%‡
6.8§
Maternal Colostrum
10
200 g (3.8 L)
<2 h & 12 h
≈22%†,§
12.1||
Santoro et al, 200421
Lifeline CS (SD)
12
100 g (2 doses)
<2 h & 12 h
22.7†
4.5†
0%†
Maternal Colostrum
12
200 g (4 L)
<2 h & 12 h
21.0†
15.7‡
100%‡
Smith and Foster, 200719
First Day Formula CS (CD)
21
100 g (2 doses)
<3 h
NR
7.5†
5%†
First Day Formula CS (CD)
21
150 g (3 doses)
<3 h
9.1†
24%†
Maternal Colostrum
21
NR g (3.8 L)
<3 h
17.6‡
95%‡
Colostrum Replacement Studies
Foster et al, 200632
Immu Start 50 CS (CD)¶
21
100 g (2 doses)
<3 h
NR
7.0†
10%†
Land O’ Lakes CR (CD)¶
21
100 g (1 dose)
<3 h
11.6‡
81%‡
Land O’ Lakes CR (CD)¶
21
200 g (2 doses)
<3 h
16.9§
95%‡
Maternal Colostrum
21
448 g (3.8 L)
<3 h
27.2||
90%‡
Swan et al, 200737
Acquire CR ± Lifeline CS (SD)
218
125 g (CR) ± 45 g (CS)
<4 h ± 12 h
NR
5.8†
7%†
Maternal Colostrum
239
291 g (3.8 L)
<4 h ± 12 h
14.8‡
72%‡
Godden et al, 200918
Land O’ Lakes CR (CD)¶
24
100 g (1 dose)
<1 h
36%†
9.6†
54%†
Land O’ Lakes CR (CD)¶
23
200 g (2 doses)
<1 h
37%†
19.0‡
100%‡
Maternal Colostrum
22
271 g (3.8 L)
<1 h
32%†
20.7‡
91%‡
Godden et al, 200922
Land O’ Lakes CR (CD)¶
24
100 g (1 dose)—Bottle
<1 h
51%†
12.5†
100%†
Land O’ Lakes CR (CD)¶
24
100 g (1 dose)—Tube
<1 h
41%‡
9.9‡
42%‡
Land O’ Lakes CR (CD)¶
24
200 g (2 doses)—Bottle
<1 h
41%‡
19.7§
100%†
Land O’ Lakes CR (CD)¶
25
200 g (2 doses)—Tube
<1 h
39%‡
18.7§
100%†
Shea et al, 200923
Land O’ Lakes CR (CD)¶
8
105 g (1 dose)
<2 h
41%†
10.7†
80%†
Land O’ Lakes CR (CD)¶
8
210 g (2 doses)
<2 h & 12 h
31%‡
14.5‡
90%‡
Brakefield et al, 201024
Calf’s Choice Total Bronze CR (CD)¶
27
200 g (2 doses)
<2 h
30%†
20.0†
100%†
Calf’s Choice Total Bronze CR (CD)¶
28
200 g (2 doses) + Probiotic
<2 h
29%†
20.1†
100%†
Morrill et al, 201025
Calf’s Choice Total Gold CR (CD)¶ + dam not fed anionic salts prepartum
10
198 g (3 doses)
<1 h & 6 h
27%†
13†
80%†
Calf’s Choice Total Gold CR (CD)¶ + dam fed anionic salts prepartum
10
198 g (3 doses)
<1 h & 6 h
26%†
13†
90%†
Poulson et al, 201060
Secure CR + Lifeline CS (SD)
146
170 g (1 CR + 1 CS)
<2 h & 12 h
NR
13.5†
71%†
Maternal Colostrum
141
NR g (3-4 L)
<2 h ± 12 h
NR
18.7‡
82%†
Place et al, 201026
Land O’ Lakes CR (CD)¶
36
150 g (1.5 doses)
<2 h
38%†
14.7†
94%†
Colostrx 130 CR (SD)
38
130 g (1 dose)
<2 h
28%‡
9.6‡
37%‡
Fidler et al, 201127
Colostrx 130 CR (SD)
22
130 g (1 dose)
<2 h
≈20%†
7.4†
14%†
Colostrx 130 CR (SD)
22
260 g (2 doses)
<2 h
≈14%‡
10.6‡
86%‡
Maternal Colostrum
22
211 g (3 L)
<2 h
≈36%§
21.4§
95%‡
Cabral et al, 201228
Calf’s Choice Total Gold CR (CD)¶
13
191 g (3 doses/1 feeding)
<1 h
32%†
15.8†
92%
Calf’s Choice Total Gold CR (CD)¶
13
191 g (3 doses/2 feedings)
<1 h & 6 h
33%†
16.8†
100%
Pithua et al, 201329
Land O’ Lakes CR (CD)¶
295
200 g (2 doses)
<6 h
27%†
15.2†
89%†
Maternal Colostrum
273
80 g (3.8 L)
<6 h
29%†
7.5‡
30%‡
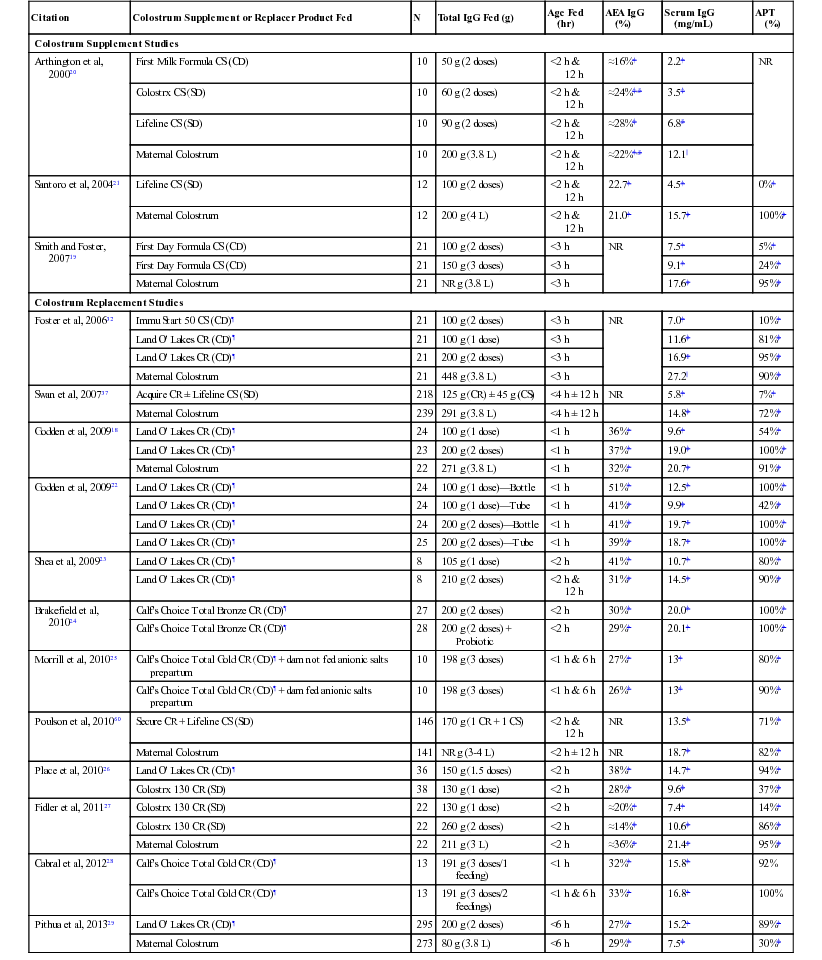
Dose of IgG Provided.
Apparent Efficiency of Absorption (AEA %) of Igg.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Colostrum and Milk Replacers
Chapter 21
