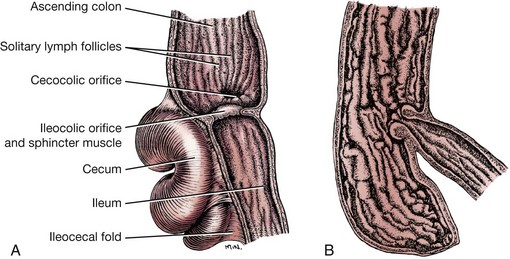
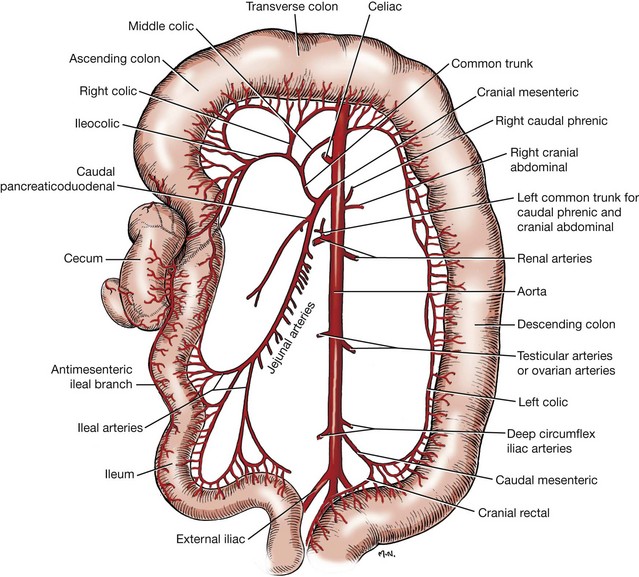
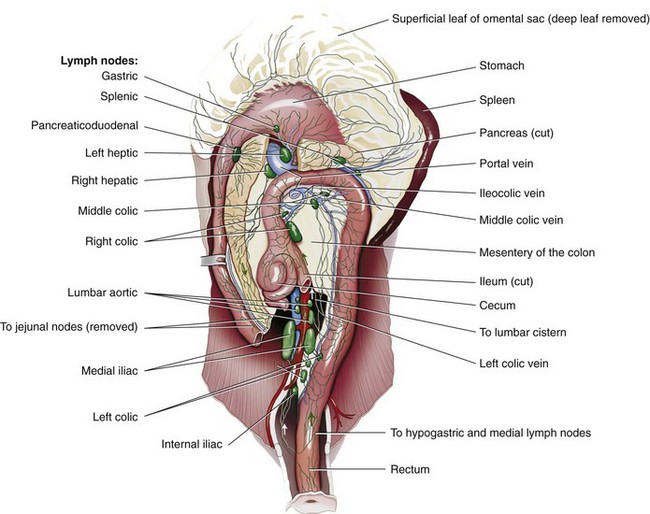
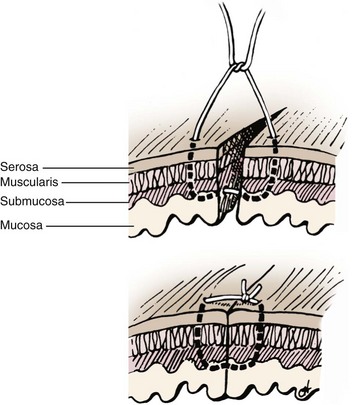
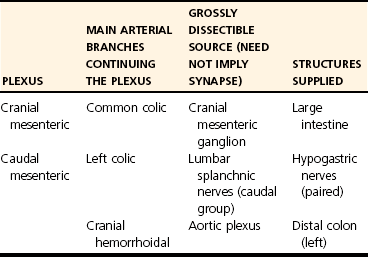
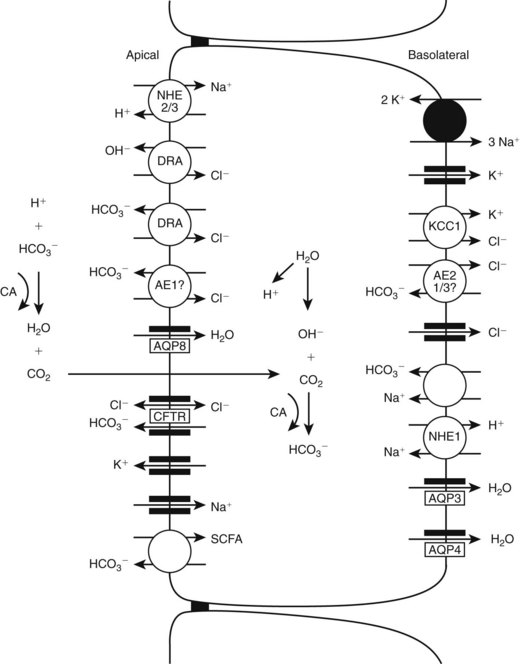
Chapter 93 The cecum and colon of dogs and cats are clearly recognizable within the abdomen. Although frequently described as the first part of the large intestine, the cecum in dogs66 and cats61 is a true diverticulum that connects to the proximal colon. Whereas the cecum is relatively long and spiraled in dogs, it is short and comma shaped in cats61 (Figure 93-1). It usually lies in a caudoventral direction or may be positioned transversely. The cecocolic orifice is approximately 1 cm distal to the ileocolic orifice in the dog, and it is adjacent to the ileocolic orifice in cats. The cecum is attached to the antimesenteric border of the ileum in dogs with the antimesenteric ileal vessels arising from the single or double ileocecal fold. The bulk of the colon receives its blood supply initially via the cranial mesenteric artery. This branches as the common colic artery, which further subdivides to supply the cecum (ileocolic artery), ascending colon (ileocolic artery proximally and right colic artery distally), transverse colon (right colic artery proximally and middle colic artery), and proximal half of the descending colon (middle colic artery). The distal half of the descending colon is supplied by the left colic branch of the caudal mesenteric artery, with the most distal portion supplied by the cranial rectal artery as it courses along the mesenteric border to the rectum (Figure 93-2). Serous membranes have a very rich network of lymphatic capillaries (lacteals) that play an important role in reabsorption of serous fluid from the body cavities. Subserous and submucosal lymphatic plexuses are present in the digestive tract, although their arrangement is different in various segments of the digestive tract.91,194 Colonic afferent lacteals drain into the right, middle, and left colic lymph nodes (Figure 93-3). Autonomic innervation to the colon is from the cranial and caudal mesenteric plexuses, which travel with the mesenteric vasculature to the intestine (Table 93-1). Table • 93-1 Autonomic Plexuses of the Abdominal Region Modified from Evans HE, de Lahunta A: Miller’s anatomy of the dog, ed 4, St Louis, in press, Saunders/Elsevier. As with the small intestine, colonic muscle and mucosal layers are relatively thick and identifiable by ultrasonography.159 Colonic layers, from inside to out, include mucosa, submucosa, muscularis, and serosal layers. Colonic mucosa is composed of columnar and cuboidal epithelial cells arranged in parallel crypts and interspersed with numerous goblet cells. A third morphologically distinct cell type, enterochromaffin cells, accounts for approximately 5% of the cellular population. Colonic mucosa differs significantly from small intestinal mucosa in that there are no villi or aggregated lymph nodules. Instead, relatively large, elevated solitary lymphoglandular complexes, through which the colonic glands discharge, are found within the mucosa. These are approximately 3 mm in diameter; in cats, they are only found within the cecum. Within the lamina propria is a double layer of lymphatic channels—one near the mucosal surface and the second just above the muscularis mucosae. Some lymphatics penetrate into the submucosa; these have prominent valves and form collecting lymphatic trunks that arise parallel to blood vessels.92 The submucosa is the supporting layer of the colon (Figure 93-4). In addition to its connective tissue, the submucosa has a rich supply of nerve vessels and lymphatics running through it. The muscularis is a double layer composed of an inner circular layer and outer longitudinal layer of smooth muscle. The serosa is connective tissue covered by a mesothelial layer, the visceral peritoneum, which is a continuation and reflection of the parietal peritoneum. The key functions of the colon include storing fecal material, acting as a reservoir for the colon’s complex essential microbial ecosystem, and maintaining fluid and electrolyte balance. The epithelium acts as an effective barrier to bacteria and macromolecules and plays an essential role in conserving water and sodium and chloride ions. Physiologic functions of the colonic mucosa include reabsorption of water, Na+, Cl−, and short-chain fatty acids, a byproduct of bacterial fermentation, and secretion of K+, HCO3−, and mucus.9,118, 219 Surface and crypt cells can perform secretory and absorptive functions, and these functions may occur simultaneously. The ability of the colon to absorb and secrete is segmental, with most of the absorption occurring in the proximal colon. The distal portion of the colon is primarily involved in fecal storage but is important in modulation of fecal water content. Colonic mucosal epithelium is a typical electrolyte-transporting epithelium, being able to move large quantities of water and sodium and chloride ions from the luminal side to the vascular side and vice versa (Figure 93-5). Electrical gradients are used as driving forces for this salt and solute transport mechanism across cells, and the gradients are established by the basolateral sodium pump (Na+, K+, ATP-ase) and apical brush border K+ selective ion channels, which hyperpolarize the plasma membrane, allowing electrically driven transport to occur. Figure 93-5 Schematic model of the cellular absorption and secretion of electrolytes and water in the mammalian colon. Examples of ion transport mechanisms are demonstrated in a single enterocyte. AE, Anion exchanger; AQ, aquaporin; CA, carbonic anhydrase; CFTR, cystic fibrosis transmembrane regulator; DRA, proteins downregulated in adenoma; KCC1, KCl cotransporter; NHE, Na/H exchanger; SCFA, short-chain fatty acid. (From Kivelä A: Carbonic anhydrase in normal and neoplastic gastrointestinal tissues with special emphasis on isoenzymes I, II, IX, XII, and XIV. Available at http://herkules.oulu.fi/isbn9514270010/html/c1390.html#AEN1392.) To maintain physiologic excretion of sodium chloride into the feces, under physiologic conditions, the colon absorbs up to 1.5 L/d of fluid. The mechanism of ion transport in the colon has been recognized for decades,48,88,118 but it is only more recently that the responsible transport proteins have been identified. Within either the apical (luminal) or basolateral cell membrane, colonic epithelial cells have a number of ion channels, carriers, and pumps that allow the transport system to work efficiently. Colonic absorption of solutes may occur by either electroneutral or electrogenic pathways. The proportion of each appears to be species specific, and further research is required to identify the ratio in the dog and cat. The ratio would also seem to vary with hormonal influence on the epithelium; for instance, aldosterone increases the activity of amiloride-sensitive Na+ channels. The result of this regulatory ion transport mechanism is the net absorption of electrolytes under normal physiologic conditions. However, colonocytes are able to switch from absorption to secretion when stimulated by secretagogues. Disruption of this balance of ion transport by disease can either lead to net secretion and diarrhea or excessive absorption and constipation. Net movement of water across the colon is osmotically driven by active absorption and secretion and occurs via paracellular and transepithelial transport routes.188 The proportion of water transport that occurs via the paracellular route, and how much is transported via epithelial cells is uncertain.188 Water channel proteins aquaporins have recently been identified; it is postulated that the presence of the isoform aquaporin-4 in the basolateral membrane of colonocytes may play an important role in fluid absorption.88,118,130 It is, however, uncertain if the aquaporin water channels are essential for colonic water absorption. It has also been suggested that the cystic fibrosis transmembrane regulator Cl− selective channel has an impact on water transport; this role is currently controversial and remains to be convincingly demonstrated. Although it has been unclear how water is absorbed against the high osmotic gradient produced by high effective osmolality of feces, the recent recognition that active fluid absorption can occur in the crypts makes it easier to reconcile this fact. Potassium ions are secreted via two types of channel in the apical membrane; channel activity is known to be increased by aldosterone and glucocorticoids. Intracellular potassium levels are maintained by the presence of two potassium channels in the basolateral membrane that are triggered by increases in intracellular Ca2+ or cyclic adenosine monophosphate.74,89,171 Absorption and secretion by colonocytes is regulated by a complex interaction of endocrine autocrine, paracrine, and neuronal stimuli. The interaction and interrelationship of the numerous secretagogues involved is complex and outside the scope of this chapter. Any alteration to this essential homeostatic mechanism will lead to either serious excessive secretions, with resultant diarrhea, or excessive absorption and constipation.74,89,118,174 Short-chain fatty acids are products of colonic bacterial fermentation of dietary fiber. Short-chain fatty acids are absorbed by colonic epithelium in parallel with NaCl. Short-chain fatty acid are metabolized by colonocytes and have a marked trophic effect on epithelium. The short-chain fatty acids butyrate, acetate, and propionate are absorbed by paracellular paths and by nonionic diffusion. As well as being cellular nutrients, short-chain fatty acids stimulate Na+ absorption by a combination of acidification (because of their cationic nature) and activation of apical membrane Na/H. They also stimulate increased HCO3− production and hence increased Cl− absorption. The increase in secreted HCO3− is important in the regulation of colonic luminal pH. Short-chain fatty acids are also important in preventing colonic irritation by reducing ionization of bile acids and long-chain fatty acids. Feeding of a nonfermentable fiber source leads to production of less short-chain fatty acids and, because the feces have increased dry matter content, tends to lead to constipation.74,164,169,174 The distal colon is the main site for fecal storage in dogs and cats, and its ability to carry out this function has an important role in maintaining fecal continence. Colonic transit is slow to ensure that fecal contents are mixed.90,185 This is achieved by a combination of segmentation and propulsive movement,31 and in the proximal colon of cats, by retrograde peristalsis.115 Segmentation is the result of coordinated circular contractions that may move either orally or aborally and is the major movement seen in the midcolon in both the dog and the cat. In the distal colon, motor activity is a combination of segmentation and propulsive aboral peristalsis.90,185 The cecum and colon are well supplied with gut-associated lymphoid tissue. This includes mononuclear cells distributed throughout the large intestinal lamina propria and mononuclear cell aggregates that have follicular and parafollicular zones. Cecal gut-associated lymphoid tissue aggregates in the cecal apex in the cat and at the ileocecal orifice of the dog.49 As with the remainder of the gastrointestinal tract, the immune system of the colon has been described as having innate and adaptive components, although immunologists have recently suggested that this is an oversimplification of the immune response, with cells not fitting neatly into such a bimodal model.16 The defense mechanism of the colon must be able to respond to potentially pathogenic antigens; at the same time, however, it needs to be able to discriminate and not react to the abundance of food and commensal bacterial antigens to which it is exposed. Antigenic stimulus provided by some commensal bacteria is important in modulating the immune response.97 As part of the innate system, a major contribution to immunity is the barrier created by colonocytes that prevents interaction between luminal material and cells lining the gut. The epithelium provides impermeability; it rapidly renews, is constantly moving (segmentation and propulsion), and is protected by mucus and a multitude of molecules with antimicrobial activity, such as cryptdins (α-defensins), lysozyme, phospholipases, and chemokines.56,218 This antimicrobial layer aids in reducing bacterial numbers and prevents colonization and mucosal invasion. The barrier’s static response is enhanced by external signals, which induce the increased expression of antimicrobial molecules and mobilization of phagocytic cells. The adaptive component of the colonic immune system gives a more specific and longer term response in that it has memory, allowing rapid elimination of any pathogen that is re-encountered. Mucosal immunity is compartmentalized,16,17,56 with the mucosa having distinct inductive and effector sites. Key to an effective adaptive response is presentation of the antigen to lymphoid follicles and to specialized sampling cells—microfold cells (M-cells) and dendritic cells (D-cells). Priming of these sampling cells leads to maturation of B- and T-cells that will migrate to the lamina propria, where they function as part of the defense system. Microfold cells of the colon differ from their neighboring colonocytes in that they lack microvilli and membrane-associated hydrolytic enzymes and produce proinflammatory chemokines and cytokines. M-cells move proteins, viruses, bacteria, and noninfectious particles transepithelially to the subepithelial lymphoid cells. The distinctive feature of M-cells is an invagination at the basolateral membrane, forming an intraepithelial pocket where memory T-cells interact with naïve and memory B-cells and D-cells interact with M-cells. After they are activated, D-cells migrate to the mesenteric lymph nodes, where they prime T-helper-1 (Th-1) cells and induce IgG and IgA production by B-cells,105,152,165 leading to colonic inflammation. D-cells may also penetrate epithelium and directly sample luminal antigens. A further cell type that is important in the colonic immune system is the intraepithelial lymphocyte. These are lymphocytes that have migrated to above the basement membrane and are found between colonocytes.92,218 They are predominantly effector memory cells with γ and δ T-cell receptor chains, although αβ T-cell receptor cells and those that lack co-receptor expression are also recognized. The unique feature of intraepithelial lymphocyte cells is their ability to express CD8αα, a characteristic of intestinal mucosal T-cells. Intraepithelial lymphocytes are thought to play an important role in epithelial homeostasis, cancer surveillance, and defense against pathogens. Paneth cells that produce antibacterial factors, such as α-defensins, in the epithelial crypts of the small intestine are not found in the colon.92,218 The aims of colonic surgery are to close the colonic wound as atraumatically as possible and to achieve tissue apposition that will maximize healing potential, minimize the risk of luminal leaking and scarring after surgery, and have minimal effect on colonic function. Historically, there have been greater concerns regarding colonic healing than any other part of the gastrointestinal tract96 because the effects of wound breakdown are potentially devastating. For this reason, surgical closure of a colonic wound requires a thorough understanding of the healing process so that the most appropriate technique is used. Failure to maximize healing potential can lead to life-threatening complications. In humans, the estimated leak rate after colonic surgery is 7% to 20%,133,198 although some older studies have reported leakage in up to 40% of cases.177 Concerns previously raised over the experimental data available on colonic healing still stand.96 These concerns include the fact that bursting strength experiments did not mimic clinical, in vivo conditions and that many of the experiments on collagen synthesis did not accurately measure collagen content. We must therefore extrapolate and use their conclusions with care. The proliferative phase occurs from days 3 and 4 to day 14. Fibroblasts proliferate, becoming the major cell type by day 4. Fibroblasts are key to clot replacement by initially producing immature collagen. During this stage, type III collagen accounts for 30% to 40% of the developing granulation tissue; in normal tissue, it accounts for only 20%, with the majority of the remainder being type I. This process is driven by a number of cytokines, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and basic fibroblast growth factor (FGF). A detailed description of the interaction between the cytokines and metalloproteinases involved is outside the scope of this chapter; readers are referred elsewhere for a comprehensive review of these processes.98 Angiogenesis occurs during the proliferative phase and is essential in oxygenating the wound and delivering nutrients and co-factors for hydroxylation of lysine and proline, key amino acids required for collagen synthesis. During this phase, there is a rapid gain in wound-bursting strength; strength is near normal by days 10 to 17 after wounding. The basic pattern of wound healing is the same for all tissues. In the colon, additional consideration must be given to the individual layers (mucosa, submucosa, muscularis, and serosa).62,198 The mucosal layer heals by cellular epithelial hyperplasia and migration. With mucosal apposition, epithelial migration is complete within 3 days, creating a seal. As noted above, the submucosa is the supporting layer of the colon because of its high collagen content, which is approximately 68% type I, 20% type III, and 12% type V. A key factor in colonic healing is that collagen is produced by the submucosa and by smooth muscle cells. Initially, strength at the wound site is weak; this weakness lasts for a minimum of 3 to 4 days, with a subsequent risk of dehiscence. Weakness results from collagen degradation by matrix metalloproteinases.18 Collagenolysis appears to occur in the narrow sutured zone of the colonic wound.1 The activity of matrix metalloproteinases decreases after day 3, at which point collagen synthesis and wound strength start to increase rapidly.1 A prospective study in humans has shown191 that patients who experienced dehiscence had a lower ratio of collagen type I to III than those who healed normally and had increased levels of matrix metalloproteinases in mucosal and submucosal layers. This evidence suggests that greater care must be taken when closing a colonic wound because dehiscence may be more likely in the first 3 to 4 days after wounding. Colonic wound strength is around 30% of normal strength after 48 hours.199 Return of strength, which reaches about 75% of normal strength at 4 months after injury, is slower in the colon than the small intestine. Factors that have been shown clinically and experimentally to have a detrimental effect on colonic wound healing are divided into local and systemic categories (Table 93-2). Local factors include hypoperfusion, poor wound apposition, wound tension, infection, and distal obstruction. Systemic influences that negatively affect healing are hypovolemia; recent blood transfusion; icterus; use of chemotherapeutic agents, such as cisplatin; immunodeficiency; and poorly controlled diabetes mellitus. Experimentally, deficiencies in zinc and iron (both co-factors required for protein synthesis) result in reduced fibroblast activity and poor collagen production. Infection leads to prolongation of the lag phase of healing and increased local levels of proteases, which delay reepithelialization and collagen formation. Disease processes, such as diabetes mellitus, hyperadrenocorticism, and hypothyroidism, are known to have profound effects on healing pathways at a cellular level, but their influence on clinical colonic healing is unproven. In a recent human review of anastomosis failure, hyperadrenocorticism and hypothyroidism were found to be nonsignificant variables.60 Colonic tissue perfusion must be maintained perioperatively to ensure adequate oxygen and nutrient delivery to the surgery site; therefore, every attempt should be made to correct hypovolemia. Local tissue ischemia can also be created or exacerbated by the surgeon, and it is essential that this is minimized. Colonic vascular supply must be preserved and tension across the wound minimized, particularly when colonic anastomosis is carried out. Care must be taken in preserving the mural vascular network as well as the segmental blood supply. Tension across the wound stretches the local vessels, reducing blood flow and resulting in low local oxygen tension because of induced local ischemia. When PaO2 decreases below 40 mm Hg, collagen formation will not occur. When PaO2 decreases to less than 10 mm Hg, angiogenesis and epithelial hyperplasia will fail, and the wound will not heal. Interestingly, anemia with hematocrit as low as 15% does not seem to affect healing. Administration of a blood transfusion may inhibit colonic wound healing, potentially because of impaired function or migration of macrophages in the wound. Retrospective studies in humans have shown that anastomoses failed in 51% of cases with poor vascular supply.127 Local perfusion of the wound edges can be difficult to quantify clinically. A crude assessment can be made based on the color of the colon and whether the cut edges bleed. Doppler ultrasonography and laser Doppler flowmetry may be used locally to asses flow; patients with low postsurgical blood flow, as detected with the latter modality, have an increased risk of dehiscence and leakage.65 Omental pedicle wraps (omentoplasty) have long been used to reinforce gastrointestinal wounds, and their use in veterinary surgery has been well described.99 The primary benefit of omentum is to stimulate and augment angiogenesis. Experimentally in rats, omentoplasty reduced anastomotic leak rates and increased wound burst strength.33 In a more recent study,46 however, there was no improvement in bursting pressure with the use of omentum. In a large study of more than 700 human patients, omentoplasty was shown to have no positive effect after colorectal or colonic resection.136 In fact, the authors suggested that its use was controversial because of potential risks, such as infection secondary to necrosis of the pedicled graft and late intestinal obstruction. In another study of 112 human patients,201 omentoplasty did not prevent dehiscence and leakage but did limit the extent and severity of the problem. Despite doubts, potential benefits of omentoplasty outweigh the potential risks, and it remains prudent to reinforce any colonic, ileocolic, or colorectal wound with omentum.84 Experimentally, rectus abdominis muscle flaps have been used to augment colonic repair.193 There are no clinical examples of its use, however. A number of different materials have been used over the years in an attempt to reinforce colonic anastomoses and prevent-leakage. Recently, use of commercially available porcine “small intestinal submucosal” (SIS) grafts has met with some success. SIS is an acellular, immunologically inert collagen matrix that is biodegradable. It has a positive effect on colonic wound healing, and immediate placement results in no acute stenosis, adhesions, or localized abscessation.93 Its widespread use cannot be advocated yet because there have been no long-term studies to ensure that the SIS patch graft does not contract and lead to late stricture formation. Preliminary work suggests that use of amniotic membrane as an incisional patch may be beneficial in clinical colonic surgery.206 In rats, application of amniotic membrane to a colonic wound increased bursting pressure and decreased inflammatory cell infiltration. It also increased rates of angiogenesis and fibroblast activity that, in turn, resulted in greater collagen deposition and hydroxyproline concentrations and decreased dehiscence rates. Chemical inhibition of angiogenesis leads to severe impairment of the colonic healing process.195 Conversely, application of exogenous factors may accelerate angiogenesis, resulting in neovascularization. The cytokine vascular endothelial growth factor (VEGF) shows much promise in the area of neovascularization. VEGF accelerates wound healing and, by increasing angiogenesis, produces a stronger anastomosis.104 It may be some time before VEGF is used clinically, however, because of concerns that neoplastic transformation may occur if the cytokine is used to excess.65 The most widely recommended wound closure for the colon is a single-layer, simple interrupted, appositional pattern, which is well recognized as having a low complication rate and has been recently described as “the current standard of care in veterinary surgery.”173 The technique of simple appositional suturing was popularized after the work of DeHoff et al.51 although, in reality, slight mucosal eversion always occurs. The key finding in this study was that appositional anastomosis produced less scar tissue and morbidity than inverting or everting suturing techniques. Despite slight eversion, which occurs even if the mucosa is not penetrated, simple appositional closure still provides the least reduction to luminal diameter.63 In a more recent study of enteric closure, use of a simple continuous pattern achieved better histologic alignment of layers and decreased surgical time and tissue trauma from excessive handling.215 Although inverting suture patterns are commonly used in humans, they provide no advantages over appositional patterns and may compromise the luminal diameter.41,189 The suture material and needle used for colonic tissue apposition should cause minimal trauma as they pass through the tissues and, ideally, minimal tissue reaction after the suture is in place. Preferably, colonic wounds are closed with monofilament absorbable (or, occasionally, nonabsorbable) suture material because it causes minimal trauma on passage through the tissue. If the surgeon is concerned about local infection, antibacterial-coated monofilament absorbable suture can be used.140 Doxycycline coating improves strength of the anastomotic site through its ability to inhibit matrix metalloproteinases.157 To minimize tissue trauma, a swaged-on needle should always be used; a round-bodied, taper-cut point needle is ideal. Chromic catgut should be avoided because it is absorbed by enzymatic degradation and phagocytosis, which produces mild to severe inflammatory response. Braided synthetic absorbable sutures are also best avoided because they tend to traumatize tissue through friction and drag and trap bacteria in their fibers.151 Triclosan-coated polyglactin 910 suture material has antibacterial activity but will cause microtrauma to tissue.77 Nonabsorbable monofilament suture should not be used in a continuous pattern because of the risk of future obstruction. Stapling devices can be used either to form an anastomosis or to close a bowel wound.37,116,205 Options include use of gastrointestinal anastomosis (GIA), thoracoabdominal (TA), end-to-end anastomosis (EEA), or skin staplers. The biggest disincentives to routine use of stapling devices in small animal surgery are cost, lack of experience, and size limitations of the instrument or staples. GIA staplers create a functional, rather than a true, end-to-end anastomosis with a GIA stapler also requires closure of cut ends of the bowel by hand suturing or use of a suitably sized TA stapler.205 TA staplers have also been used for closure of cecal resection sites in dogs37 and for end-to-end anastomosis of intestines. With the latter technique, an everting anastomosis is performed by firing a TA stapler across one third of the apposed end segments. The process is repeated two more times, with staple lines overlapping at each end to ensure complete apposition. End-to-end anastomosis with a TA stapler is difficult to perform on small-diameter intestine and is therefore rarely used in dogs and cats. Of greater potential benefit in colonic surgery is the tubular-shaped EEA device, which creates a true inverting anastomosis by placement of a circumferential staggered double row of surgical stainless steel staples. The EEA device can be placed transcecally, through an enterotomy, or transrectally.6,116 Stapled anastomosis with the EEA device is described later in this chapter (see Colectomy). Experimentally, use of skin staples for small intestinal anastomosis achieved good apposition of intestinal anastomosis.42 Anastomosis of large or small intestine with skin staplers has not been described clinically. The biofragmentable anastomosis ring (Valtrac, Covidien Autosuture, Norwalk, CT) is composed of polyglycolic acid and barium sulfate. The technique for colonic anastomosis with a biofragmentable anastomosis ring device is described later in this chapter (see Colectomy). Biofragmentable anastomosis ring anastomosis produces an inverted wound that has a high initial bursting strength that is maintained over a 28-day period.30 Its successful use has been described in normal experimental cats103 and clinically for the management of feline idiopathic megacolon.173 Serosal tearing appears to be a common occurrence because of the large size of the ring (available sizes are limited) but is readily rectified. The authors also reported that short-term minor complications, such as anemia and anorexia, were more common with biofragmentable anastomosis ring anastomosis than sutured colocolostomy. Sutureless closure of the colon has been described using lasers, tissue glue, or fibrin sealant. In early studies of sutureless colocolic anastomosis with a low-power neodymium-doped yttrium-aluminum-garnet (Nd:YAG) laser, bursting strength was initially lower than noted with conventional suture techniques; however, the healing process was quicker, and fewer adhesions were present.119 A longer term study suggested that use of laser for end-to-end colonic anastomosis resulted in better anatomic realignment and less fibrosis.111,112 More recently, researchers found that anastomosis using a gallium arsenide laser system, which also bonded albumin to the wound, resulted in much stronger anastomosis than sutured anastomosis.186 In that study, initial bursting strength was double that of a hand-sutured wound. Clinical use of these techniques is limited. Use of tissue glue as a means of achieving anastomosis has produced conflicting results. In one study, use of 2-octyl cyanoacrylate for colonic anastomosis in the rat achieved anastomotic strength equal to that after conventional suturing. In that study, the anastomosis was not tested clinically.108 In another study, use of n-butyl-2-cyanoacrylate in rats had a negative influence on colonic healing during the first postoperative week compared with sutures.155 Based on the available evidence, tissue glues cannot be recommended for colonic wounds at this time. Fibrin sealant is a topical hemostat, sealant, and tissue adhesive composed of thrombin and fibrinogen187 that is approved by the Food and Drug Administration (FDA) for colonic sealing in humans. Early work suggested that sutureless closure with fibrin sealant produced an anastomosis with lower bursting strength than suture closure.209 More recently, sutureless closure with a fibrin sealant increased the early bursting strength of the anastomosis.2 Similar findings have been noted experimentally in pigs: with a follow-up of up to 540 days, sutureless anastomosis produced minimal inflammation and scar tissue formation and rapid healing with no signs of necrosis or stenosis of anastomotic sites.55 Although experimental work is encouraging, to the author’s knowledge, there have been no studies of the efficacy of fibrin sealant in the clinical setting. Diagnostic Techniques for Large Intestinal Disease Survey radiographs often provide invaluable information on the size and position of the cecum and colon8 and the presence of extraluminal masses or malunion of pelvic bone fractures that may cause pelvic stenosis. Further information may be obtained by use of positive- and double-contrast radiography, allowing assessment of size70 and detection of luminal and intramural lesions. When contrast radiography is contemplated, a soapy enema should be administered 2 to 4 hours beforehand. Barium sulfate is diluted 50/50 with warm water, and 10 mL/kg is allowed to flow under gravity into the colon per rectum. Barium sulfate is best administered via balloon catheter placed per rectum. The colon and cecum should be filled with contrast; some will ascend into the small intestine. For a double-contrast study, the patient is either allowed to evacuate the bowel or the colon is drained of the barium sulfate; air is then introduced. Care should always be taken to prevent overdistention, which has the potential for perforation, particularly if there is ulceration or intramural neoplastic infiltration. Because of the presence of gas and feces within the lumen, colonic ultrasonography may be challenging. Ultrasonography of the cecum and colon is most useful in assessing the presence of intussusception or masses.120,146,156 It can also be used to image mesenteric and sublumbar (medial iliac) lymph nodes and facilitate guided biopsies45 to assess for secondary spread from a colonic mass. Use of advanced imaging modalities for the assessment of colonic disease is not well established. Enhanced imaging, such as parallel imaging with MRI scanners, has allowed experimental detection of polyps that are 5 mm in diameter.142 Possibly of greater value in the future is the use of multidetector-row CT, which allows for a virtual endoscopic examination of the intestinal tract, although it clearly has the disadvantages that tissue is not viewed directly and biopsies are not possible.223 Colonoscopy is regarded by many as the method of choice for examining patients with clinical signs of large intestinal disease and has the advantage of providing access for direct biopsy.182 Flexible endoscopes are preferred to rigid proctoscopes because they allow evaluation of the entire length of the large intestine. For all colonoscopy procedures, it is essential that the patient is prepared adequately; otherwise, the examination will be suboptimal. The patient should be fasted for 24 to 48 hours, and an oral cleansing solution should be administered on the afternoon before the procedure. Oral cleansing solutions prescribed for humans contain an osmotic laxative (polyethylene glycol) combined with electrolytes to help prevent dehydration. Because large volumes (20 to 30 mL/kg) are usually required, orogastric intubation is usually necessary for solution administration.182 Some authorities recommend a second administration 1 to 2 hours later.168 Two warm water enemas (20 mL/kg) are administered on the morning of colonoscopy, and the second is administered approximately 1 hour before the procedure. After being anesthetized, the patient is placed in left lateral recumbency, which allows any fluid in the transverse colon to drain into the descending colon. This improves visualization of the transverse and ascending colon and facilitates passage of the endoscope through the colonic flexures.182 The endoscope tip is inserted gently into the anus and advanced into the rectum, and the colonic lumen is insufflated with air. After the lumen is sufficiently distended, the endoscope is advanced, and the mucosa is examined for any visible abnormalities, which should be biopsied. If the colon is severely ulcerated, biopsies should be taken with care because perforation may occur. Preparation of the colon in dogs and cats is controversial: some authors advocate preoperative cleansing, but others suggest that there is no need for any preparation.96 The rationale for cleansing has been extrapolated from colonic surgery in humans, in whom reduction of bacterial numbers by preoperative oral antibiotics and mechanical cleansing has long been advocated as best practice. Recent prospective human studies of 380 and 329 patients166,226 showed that there was no advantage to mechanical cleansing; in fact, 46.9% of those who underwent the procedure developed complications compared with 37.6% of those who only received perioperative antibiotics. Based on these results, there is no indication for bowel cleansing in dogs and cats. Indeed, their use is counterproductive because it turns fecal material into a liquid slurry within the colon, which is much more likely to leak and contaminate the abdominal cavity during surgery.96 Use of perioperative antibiosis for colonic surgery has long been advocated in humans and small animals.39,96,148,149 Although still popular in human surgery, use of oral preparations, such as neomycin combined with erythromycin, has no clinical advantage over perioperative intravenous antibiotics. As a disadvantage, oral preparations must be administered 24 hours before surgery.19 Use of perioperative intravenous antibiotics, administered 1 hour before surgery, significantly reduces the risk of surgical site infection in colonic anastomosis in humans.100 This is in line with the recent consensus document from the U.S. Medicare National Surgical Infection Prevention Project,19 which recommends that the “first antimicrobial dose should begin within 60 minutes before surgical incision and that prophylactic antimicrobial agents should be discontinued within 24 hours of the end of surgery.”19 Choice of antibiotic is based on the likely contaminants present: coliforms and anaerobes. Cefoxitin has been recommended for parenteral prophylaxis alone; given its half-life, a repeat dose should be given hourly during the procedure.19,96 Combination parenteral treatment may include cefazolin or cefuroxime and metronidazole;26 if the procedure is prolonged, the cephalosporin should be given again after 2 hours. There is no indication for postoperative use of antibiotics after colonic surgery.
Colon
Anatomy
Vasculature, Lymphatics, and Nerves
Microscopic Structure
Physiology
Electrolyte Transport
Water Transport
Secretion
Short-Chain Fatty Acids
Fecal Storage
Immune System
Healing of the Colon
Stages of Wound Healing
Proliferative Phase
Healing of Individual Tissue Layers
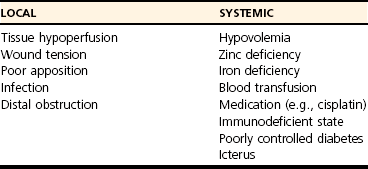
Factors That Negatively Affect Wound Healing
Tissue Perfusion
Methods for Improving Colonic Wound Healing
Vascularized Tissue Wraps
Colonic Reinforcement
Cytokines
Techniques for Colonic Wound Closure
Suture Closure
Suture Material
Staplers
Biofragmentable Anastomosis Ring
Sutureless Closure
Cyanoacrylates
Fibrin Glue
Perioperative Considerations
Radiography
Ultrasonography
Computed Tomography and Magnetic Resonance Imaging Scanning
Endoscopy
Preoperative Preparation of the Colon
Antibiotic Prophylaxis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree