CHAPTER 36 Clostridial Diseases
The Clostridium genus encompasses a wide range of commensal and opportunistic pathogens that can be found in a wide range of animal species (Table 36-1). All members of this genus are anaerobic gram-positive spore-forming bacteria. Although all are anaerobes, Clostridium spp. have variable tolerance to oxygen; some, such as Clostridium perfringens, are very tolerant, whereas others, such as Clostridium difficile, are very fastidious. Regardless, an important aspect of clostridia is their ability to exist in both vegetative and spore forms. The vegetative forms are the active forms of the organism; the spore form is dormant and highly resistant to oxygen, environmental effects, and disinfectants. Because of this, many clostridia can be found in the environment and can persist there for prolonged periods.
Table 36-1 Important Clostridial Pathogens
| Organism | Disease |
|---|---|
| C. difficile | Enterocolitis, duodenitis/proximal jejunitis |
| C. perfringens | Enterocolitis, myonecrosis, soft tissue/wound infections |
| C. tetani | Tetanus |
| C. botulinum | Botulism, grass sickness |
| C. piliforme | Tyzzer’s disease |
| C. sordellii | Enterocolitis, septicemia, acute pasture rhabdomyolysis, myonecrosis |
| C. bifermentans | Acute pasture rhabdomyolysis |
| C. sporogenes | Myonecrosis |
| C. septicum | Myonecrosis |
| C. chauvoei | Myonecrosis |
CLOSTRIDIUM DIFFICILE COLITIS
Clinical Pathology
Clinical pathology results are variable and nonspecific. There are no indicators that can be used to suggest or confirm a clostridial cause. Leukopenia and neutropenia, often with a left shift, are common. Affected animals may exhibit either hypoproteinemia resulting from protein loss or hyperproteinemia as a consequence of hemoconcentration. Prerenal azotemia is common in dehydrated animals.
Diagnosis
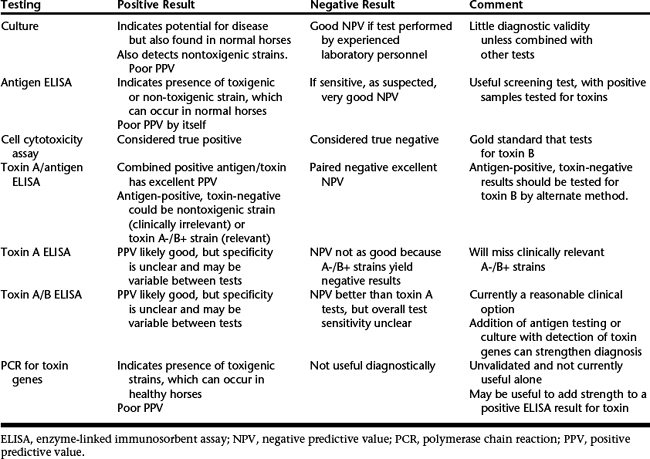
Isolation of C. difficile alone is not diagnostic because it can be found in normal horses. Detection of C. difficile toxins is the preferred approach but is not straightforward (Table 36-2). The gold standard for CDAD is detection of toxin B by use of a cell cytotoxicity assay, but this test is rarely available. Commercial enzyme-linked immunosorbent assay (ELISA) tests are available for detection of C. difficile toxin A, C. difficile toxin A or B, and “common” antigen. None of these has been validated for use in horses, and the sensitivity and specificity of these tests are variable in different animal species. Concern has been expressed about the usefulness of all these tests. Although tests are still useful clinically, their limitations should be acknowledged. Polymerase chain reaction (PCR) testing is also of limited usefulness because the presence of toxigenic C. difficile is not necessarily clinically relevant.
Treatment
Supportive care is the most important aspect of treatment. Depending on the severity of disease, this may include intravenous (IV) fluids (crystalloids or colloids), plasma transfusion, nutritional support, and measures to combat endotoxemia, such as administration of polymyxin B (1000-6000 units/kg slowly IV every 8 to 12 hours) or low doses of flunixin meglumine (0.25 mg/kg IV every 8 hours). Di-tri-octahedral smectite (1.5 kg loading dose administered orally [PO] followed by 450 g PO every 6 to 8 hours) is commonly used. It binds to clostridial toxins in vitro, although clinical efficacy is unclear. Probiotics are sometimes used, but there is minimal evidence regarding efficacy, and results of trials in humans have been mixed. Administration of the yeast Saccharomyces boulardii (1 × 1010 yeast cells PO every 12 hours for 14 days) has been reported to decrease the duration of diarrhea (but not outcome) in a small trial of horses with acute colitis. This yeast produces a protease that might be able to cleave clostridial toxins in the intestinal tract; however, further efficacy data are required. The potential role of other probiotics is unclear. If diarrhea is considered to be associated with antimicrobial treatment, the treatment should be stopped, if possible.
CLOSTRIDIUM PERFRINGENS COLITIS
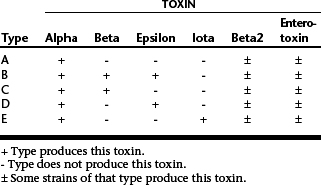
C. perfringens is a ubiquitous anaerobic spore-forming organism that is commonly found in the intestinal tract of many species and in the environment. Although strains of C. perfringens can be classified into five types by their ability to produce major toxins (Table 36-3), the role of different types in enterocolitis is poorly understood. Enterotoxin-producing strains have been evaluated most, and studies have demonstrated an association between the presence of enterotoxin in feces and diarrhea. β2-toxin–producing strains have received attention recently; this may also be an important virulence factor. Type C strains have been implicated in severe disease in foals. Type A strains are most commonly isolated from horses with diarrhea, but there is some debate over the clinical relevance of these strains in the absence of β2 or enterotoxin.
Pathogenesis and Clinical Presentation
The pathogenesis of disease is poorly understood. Presumably, disease occurs following production of one or more toxins in the intestinal tract. Whether this occurs following ingestion of C. perfringens or from proliferation of endogenous C. perfringens is unclear. The different toxin types can have different effects in the intestinal tract, and the mechanisms of action are not well defined.