Clinical Pathology
2.1 Haematology and biochemistry
As rabbits are used extensively for toxicological and physiological studies, there is a lot of information about the effects of experimental infections, drugs and toxic substances on haematological and biochemical parameters. There is also information about diseases of commercial rabbits, primarily based on post-mortem examination. Information about the effects of clinical diseases on the blood picture of live pet rabbits or the use of blood tests as diagnostic or prognostic indicators has increased exponentially over the past decade with the increasing popularity of rabbits as pets.
2.1.1 Sample collection
The collection of blood and urine samples is covered in Section 1.10.3. Parameters such as glucose, creatine kinase and aspartate aminotransferase (AST) can be altered by stress associated with handling and restraint, or tissue damage that has occurred during sample collection. For example, potassium results appear less reliable in samples taken with a plastic cannula as opposed to a hypodermic needle (Robson et al., 1981).
Rabbit blood haemolyses easily and clots quickly (Perry-Clark and Meunier, 1991). Small clots affect haematology results and haemolysis affects certain biochemistry results, especially potassium and serum inorganic phosphorus that are released from erythrocytes. Rapid clotting can affect the performance of some analysers and heparinized syringes and needles are required to avoid this.
2.1.2 Fasting and other physiological considerations
It is not possible to take a guaranteed fasting sample from a rabbit because they ingest caecotrophs. Parameters such as blood glucose are affected by digestion. Some parameters such as bile acids, cholesterol and urea follow a diurnal rhythm that also affects the total and differential white cell count (Fekete, 1989; Fox and Laird, 1970; Loeb and Quimby, 1989). Stress associated with car journeys or a period in unfamiliar surroundings will increase blood glucose and alter haematological parameters such as the distribution of neutrophils and lymphocytes. Pregnancy affects parameters such as protein, haematocrit, cholesterol, alkaline phosphatase, triglycerides (Viard-Drouet et al., 1984), glucose, sodium, calcium, phosphate and red cell indices (Palm, 1997). Serum cholesterol is the parameter most affected and can be up to 30% lower in pregnant than in non-pregnant animals (Palm, 1997).
Anaesthesia affects some blood parameters such as potassium (Robson et al., 1981). The effect of anaesthesia on biochemical parameters is minimized by taking samples within 5 minutes of induction. Intravenous or intraosseous fluids will also affect haematological findings (Ros et al., 1991) and blood samples should be taken prior to treatment.
2.1.3 Reference ranges
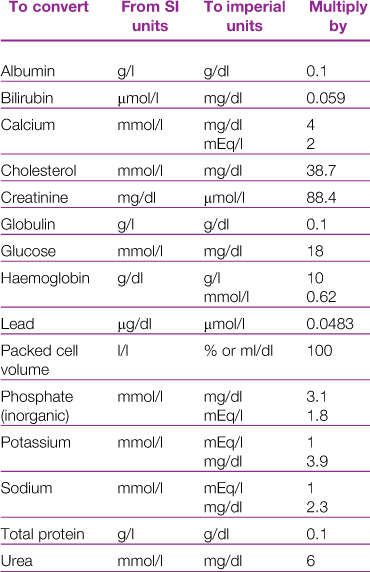
There are a number of published reference ranges for haematological and biochemical parameters in rabbits. Conversion factors for the variety of units used in reference ranges are given in Table 2.1. Differences in analytical techniques between laboratories can lead to disparity in results. Historically laboratory data were often derived from populations of rabbits of the same breed, sex and age that are not genetically diverse. In contrast, the pet rabbit population is made up of a variety of breeds and cross-breeds, and is composed of rabbits of all ages. With the increased interest of rabbit owners in pursuing diagnostics for their pets, many commercial labs have built up a vast database of samples from a genetically heterogeneous population. Significant breed and sex differences have been noted for some parameters in laboratory rabbits (Kozma et al., 1974).
Published reference ranges for pet rabbits are derived either from sets of data from laboratory rabbits or from data collected by the author. Some reference ranges are an amalgamation of other reference ranges often without addressing the fact that the ranges have been determined using different methodologies. This results in ranges so wide that almost any result will fall within the normal range. For example, the reference range for serum albumin concentrations of pet rabbits is given as 27–46 g/L (Malley, 1996) based on four published sets of data. Gillett (1994) gives an even wider range of 27–50 g/L for laboratory rabbits based on five sets of data. For some parameters there are big differences between published reference ranges. An example is blood calcium. Gillett (1994) gives a range of 1.5–3.4 mmol/L, compared to 3.4–4.0 mmol/L given by Harkness and Wagner (1995). Differences in calcium content of the diet between the groups of rabbits or differences in analytical technique could explain these discrepancies. Different laboratory methods can result in large differences between reference ranges. An example is alkaline phosphatase. Collins (1988) gives a reference range of 4.1–6.2 IU/L, compared to 10–70 IU/L (Gillett, 1994) or 112–350 IU/L (Harkness and Wagner, 1995).
Automated flow cytometers are designed to measure numbers of human blood cells. Rabbit erythrocytes are smaller in diameter than human erythrocytes and also vary in diameter. These differences cause problems with some automated analysers. Automated differential white cells counts cannot be relied upon for rabbit blood and accurate results can only be obtained using manual counting methods (Kabata et al., 1991).
2.2 Haematology
2.2.1 Morphological characteristics of blood cells
Some morphological characteristics of rabbit blood cells are different from other species. The red blood cells vary in diameter within a range of 5.0–7.8 μm (Sanderson and Phillips, 1981). This variation in diameter (anisocytosis) is a feature of blood smears from rabbits and is not a significant finding (see Figure 2.1). The red cell distribution width (RDW) is a measurement of the variation in size of erythrocytes and is higher in rabbits (11–15, IDEXX reference range) than in dogs and cats (8–10; Bush, 1991). In dogs and cats, anisocytosis is indicative of the presence of reticulocytes and a regenerative anaemia. In rabbits, 1–4% of circulating erythrocytes may be reticulotytes. Polychromasia and reticulocytes in rabbit blood smears have been attributed to the short life span and high turnover of erythrocytes (Kraus et al., 1984). Nucleated red cells and Howell–Jolly bodies can also be found occasionally (McLaughlin and Fish, 1994).
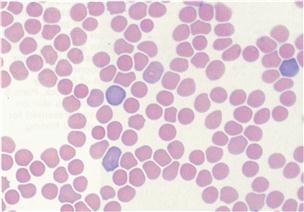
Figure 2.1 Anisocytosis and polychromasia.
The red blood cells of rabbits vary in diameter within a range of 5.0–7.8 μm. This variation in diameter (anisocytosis) is a feature of blood smears from rabbits and is not a significant finding. There is also variation in erythrocyte colour (polychromasia) in normal rabbits. The blood smear illustrated was from a normal rabbit and was stained with Rapi-diff. Interpretation of haematology results is discussed in Section 2.2.2. Photograph taken by Dr Joan Duncan, IDEXX Laboratories, Wetherby.
Rabbit neutrophils have an almost colourless cytoplasm and contain two types of granules. The smaller granules stain pink, giving a pinkish colour to the cytoplasm. Larger granules stain a deeper pinkish-red. The overall colour of the neutrophils varies according to the proportion of large and small granules. The granular appearance of the cytoplasm has led to different nomenclature. Rabbit neutrophils may be called heterophils, pseudoeosinophils, acidophils or amphophils, depending on the text (Benson and Paul-Murphy, 1999; Sanderson and Phillips, 1981). Neutrophils measure 10–15 μm in comparison with eosinophils, which measure 12–16 μm (Sanderson and Phillips, 1981).
Small lymphocytes are seen more commonly than large lymphocytes. The average cell diameter for small lymphocytes is 7–10 μm (Cooke, 2000). The lymphocytes are round cells with the typical morphology described for other species. An occasional large lymphocyte may be seen in the circulation or normal rabbits. These cells have a few azurophilic granules in the cytoplasm (Jain, 1986). Activated lymphocytes (immunocytes) typically have a more intensely stained cytoplasm.
Monocytes are large nucleated cells measuring 15–18 μm (Cooke, 2000). The nucleus has a diffuse lacey chromatin pattern that lightly stains a purple blue. The vacuolar cytoplasm stains light blue.
Eosinophils can be distinguished from neutrophils by their greater size and large acidophilic granules.
In contrast to other laboratory species, basophils are frequently found in the circulation of rabbits in small to modest numbers (Jain, 1986).
2.2.2 Interpretation of haematology results
A reference range for haematological parameters is given in Table 2.2. The haematological picture gives an indication of the general health status of a rabbit. Stress and a range of diseases will alter haematological parameters. Hinton et al. (1982) analysed the haematological findings in 117 healthy and diseased rabbits and found that blood cellularity was a good indicator of disease especially with regard to erythrocytes (these tended to be depressed) and lymphocyte counts (the counts were variable; however, heterophils tended to become dominant in acutely ill animals). These findings are in agreement with studies of experimental infections in rabbits (Toth and Krueger, 1988, 1989) and in a clinical study by Harcourt-Brown and Baker (2001). In this study, significantly higher red cell counts, haemoglobin values, haematocrits and lymphocyte counts were found in rabbits kept outside with unlimited access to grazing and exercise. A comparison was made with rabbits kept in hutches and those suffering from dental disease.
Table 2.2
Haematological reference range
| Laboratory reference range (Gillett, 1994) | Comment | |
| Erythrocytes | 4–7 × 1012/L | Anisocytosis, polychromasia, small numbers of nucleated red blood cells and Howell–Jolly bodies can be normal findings in rabbit blood films. |
| Haemoglobin | 10–15 g/dL | |
| PCV | 33–48% (0.33–0.48 L/L) | 31–40% usual range for pet rabbits |
| MCV | 60–75 μm3 (fL) | |
| MCH | 19–23 pg | |
| MCHC | 34.5 g/dL | |
| Reticulocytes | 2–4% | Small numbers normal, increased with regenerative anaemia. |
| Platelets | 250–600 × 10–3/L (10–9/L) | |
| White cells | 5–12 × 10–3/L (10–9/L) | Total white cell count usually fairly stable. Increase indicates significant insult. |
| Neutrophils | 30–50% | Neutrophil:lymphocyte ratio is approximately 1:1 in a healthy adult; this increases in acute infection. |
| Lymphocytes | 30–60% | Lymphocyte numbers are depressed in acute infection, thereby increasing the neutrophil:lymphocyte ratio. Small numbers of large lymphocytes are normal in the circulation; activated lymphocytes are not. |
| Eosinophils | 0–5% | Not consistently elevated with parasitism. |
| Basophils | 0–8% | Significance of a decrease is not known. |
| Monocytes | 2–10% | Often found elevated in animals with chronic disease. |
From: Gillett, C.S. (1994). Selected drug dosages and clinical reference data. In The Biology of the Laboratory Rabbit. 2nd edn. (P.J. Manning, D.H. Ringler, C.E. Newcomer, eds). pp 467–472. Academic Press.
2.2.3 Red cell parameters
Reference ranges for packed cell volumes (PCV) vary between sources, with values between 30 and 50% (Malley, 1996). Pet rabbits tend to have PCV values at the lower end of the range, typically between 30 and 40% (Harcourt-Brown and Baker, 2001). Values greater than 45% are indicative of dehydration, especially in rabbits suffering from gut motility problems. Values of less than 30% indicate anaemia that can be classified into non-regenerative and regenerative in a manner similar to that of other species.
Regenerative anaemia is associated with chronic blood loss, where compensatory mechanisms are attempting to redress the balance. Common causes include heavy flea infestation or from sites that intermittently bleed such as a uterine endometrial aneurysm or uterine adenocarcinomas, a common finding in middle-aged unneutered does. Lead poisoning can result in a regenerative anaemia with the presence of nucleated erythrocytes, hypochromasia, poikilocytosis and cytoplasmic basophilic stippling (Fudge, 2000). Non-regenerative anaemia is caused by diseases such as lymphoma or chronic renal failure. Autoimmune haemolytic disease has not been described as a clinical phenomenon in pet rabbits although there are reports that it occurs. Autoimmune haemolytic anaemia has been reported in laboratory rabbits in association with lymphosarcoma (Weisbroth, 1994). Chronic debilitating disease such as dental disorders or abscesses often cause a mild anaemia in pet rabbits (Harcourt-Brown and Baker, 2001).
Nucleated red blood cells can be associated with acute infectious processes although a few may be present in normal blood films. Experimentally, infections with Escherichia coli and Staphylococcus aureus cause an increase in number of circulating nucleated red blood cells during the septicaemic phase of the disease (Toth and Krueger, 1988, 1989).
2.2.4 White blood cells
2.2.4.1 Total white blood cell count
There is a natural diurnal variation in total white cell count, with lowest counts occurring during the late afternoon and evening (Fox and Laird, 1970). The white cell count also varies with age (Jain, 1986). It is higher in young rabbits of approximately 3 months of age and in adults over 1 year old. The first peak in leucocyte count is due to an increase in the number of lymphocytes. The second peak is due to an increase in the number of neutrophils.
In other species, an increased white blood cell count is seen in response to bacterial infection or in response to endogenous or exogenous corticosteroids. Rabbits do not develop marked leucocytosis after either acute infectious challenge or the intramuscular injection of cortisone acetate (Toth and January, 1990). If an elevated total white cell count is found, this should be confirmed, and if consistent, it should be assumed to be significant, as it is likely that a significant disease process is occurring. In two studies by Toth and Krueger (1988, 1989) controlled experimental infections with S. aureus, Streptococcus pyogenes, E. coli and Candida albicans resulted in fever, increased plasma cortisol concentrations, neutrophilia and lymphopenia but no significant increase in total white blood cell count. High white cell counts can be found in rabbits suffering from lymphosarcoma (McLaughlin and Fish, 1994). Low white blood cell counts can be found in association with chronic disease (Hinton et al., 1982).
2.2.4.2 Differential white cell counts
Although the total white cell count of rabbits seldom alters in diseased rabbits, the differential white cell count may show a number of changes due to the redistribution of white blood cells. A feature of many diseases in rabbits is an alteration in the ratio of neutrophils to lymphocytes and a reduction in blood cellularity (Hinton et al., 1982). The neutrophil:lymphocyte ratio has been suggested as a method of predicting whether a rabbit is normal or abnormal (McLaughlin and Fish, 1994). Jain (1986) described a physiological variation in the neutrophil:lymphocyte ratio according to the age of the rabbit. The ratio changes from 33:60 in the second month of life to 45:45 in rabbits over 1 year of age. Stress and increased cortisol levels, as well as disease, can affect this ratio.
2.2.4.3 Effect of stress on the differential white cell count
Stress alters the differential white cell count in any species. Rabbits are particularly susceptible to the effects of stress. A car journey to the surgery, a period in the waiting room next to a barking dog or the excitement of handling can be reflected in the blood picture. Adrenaline and cortisol affect the distribution of lymphocytes throughout the body. Administration of exogenous adrenaline to rabbits results in redistribution of lymphocytes from spleen and bone marrow to peripheral blood, lungs and liver (Toft et al., 1992a). Conversely, exogenous corticosteroid administration results in a redistribution of lymphocytes from the peripheral blood, bone marrow and spleen to the lymphatic tissue in rabbits (Toft et al., 1992b). Prolonged periods of stress cause neutrophilia and lymphopenia. Marked changes in white cell distribution with a relative neutrophilia and lymphopenia were found in a study of cortisol levels and haemograms of rabbits after transport, either by air or by lorry. The changes in white cell distribution lasted for 24–48 h and were correlated with increased cortisol levels (Toth and January, 1990).
Disease is stressful as well as having a direct effect on the production and distribution of white cells.
Rabbits with experimental infections exhibit a neutrophilia and lymphopenia in comparison with control rabbits handled and sampled in exactly the same manner but inoculated with heat-killed cultures (Toth and Krueger, 1989). Rabbits inoculated with a heat-killed culture do not experience the same rise in plasma cortisol concentrations as those inoculated with a live culture, indicating that the stress response is initiated by disease rather than by handling. Therefore the stressful effects of a long car journey to the surgery or a morning spent in a kennel next to a barking dog is more likely to affect the neutrophil:lymphocyte ratio than the excitement response of taking blood.
2.2.4.4 Neutrophils
Neutrophils function primarily as phagocytes and are important in infectious conditions and in inflammation. Rabbit neutrophils are often called heterophils in the literature; this is due to the staining characteristics of the cytoplasmic granules, and is not due to a difference in function. In other species, a neutrophilia occurs in response to inflammation, especially bacterial infection. An increase in the number of circulating neutrophils causes a rise in total white blood cell count. This response is not marked in rabbits. However, a change in the distribution of white cells can occur in response to infection with a relative neutrophilia and lymphopenia but no alteration in total white cell count (Toth and Krueger, 1989). A mature neutrophilia accompanied by an increase in plasma cortisol can also be associated with stress (Toth and January, 1990).
2.2.4.5 Lymphocytes
Lymphocytes are involved in immunological responses and are distributed throughout the body in various tissues, including blood, bone marrow, lymph nodes, spleen and gut-associated lymphoid tissue. The number of lymphocytes in the blood reflects a balance between cells leaving and entering the circulation and does not necessarily reflect a change in lymphopoiesis. Increased cortisol levels cause a lymphopenia and increased adrenaline levels cause lymphocytosis (Toft et al., 1992a,b).
In rabbits, lymphopenia is a feature of a variety of clinical diseases, including vestibular disease, gut impaction and abscessation (Hinton et al, 1982). Marked lymphopenia has been reported as a feature of differential white cell counts of pet rabbits, especially those suffering from dental disease (Harcourt-Brown and Baker, 2001).
Lymphoma is a relatively common tumour of rabbits and atypical lymphocytes can be found in the peripheral blood of some of these patients. These lymphocytes contribute to an elevated total white cell count in some cases.
2.2.4.6 Eosinophils
The main function of eosinophils is detoxification by either inactivation of histamine or histamine-like toxic materials. Eosinophils are important in the allergic response and are capable of phagocytosis (Kerr, 1989). Chronic eosinophilia can be seen in diseases of tissues that contain large numbers of mast cells such as the skin, lungs, gastrointestinal tract and uterus. Eosinophilia can be associated with parasitism, especially when parasites are migrating through tissue. Mild eosinophilia has been associated with experimentally induced chronic ascarid parasitism in rabbits (Gupta and Trivedi, 1981). However, heavy worm burdens are rare in pet rabbits. Encephalitozoonosis does not appear to cause an eosinophilia. Slight to moderate elevations in eosinophil counts can be observed after traumatic wound repair in rabbits (Fudge, 2000).
Although eosinopenia can be a significant finding in other species, low eosinophil counts or a zero count are not unusual in rabbits.
2.2.4.7 Basophils
Basophils are similar to neutrophils but have dark blue cytoplasmic granules. Although basophils are rare in blood films from species such as the dog, they may be seen commonly in rabbit blood (Kerr, 1989). Basophil counts as high as 30% have been reported in clinically normal animals (Benson and Paul-Murphy, 1999).
2.2.4.8 Monocytes
In other species, monocytosis is associated with chronic disease, particularly chronic inflammatory conditions. In rabbits, increased monocyte counts can be associated with chronic bacterial infection. Hinton et al. (1982) noted increased monocyte counts in rabbits with subcutaneous abscesses, mastitis and ‘labyrinthitis’. However, monocyte counts within the laboratory reference do not signify the absence of chronic infection. Rabbits with chronic osteomyelitis due to dental disease can have monocyte counts within the laboratory reference range (Harcourt-Brown, unpublished data).
2.3 Biochemistry
A reference range and discussion of relevance of abnormal results for biochemical parameters is given in Table 2.3.
Table 2.3
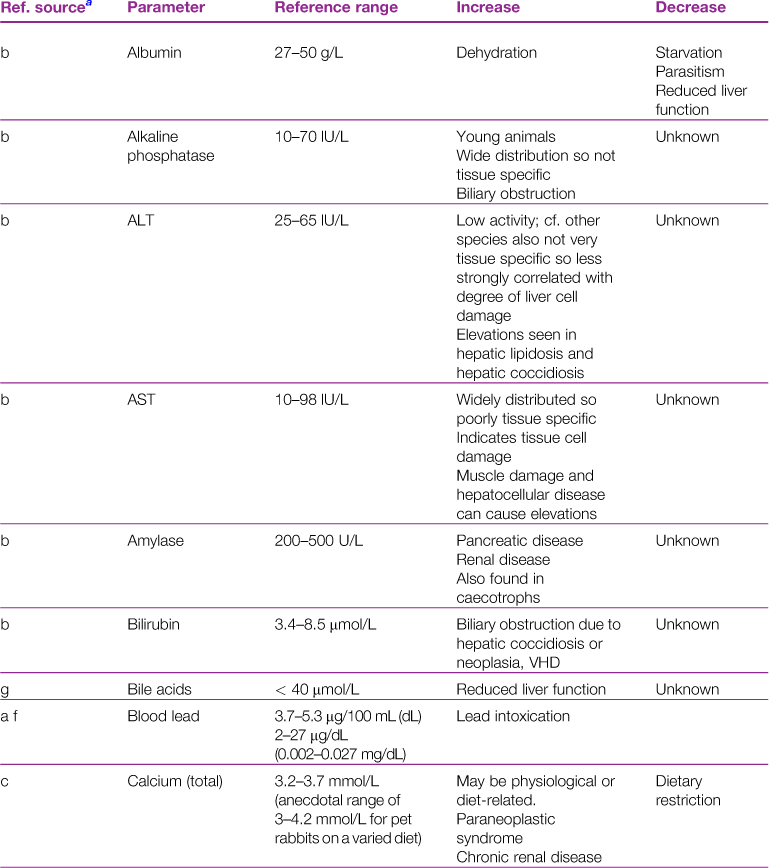
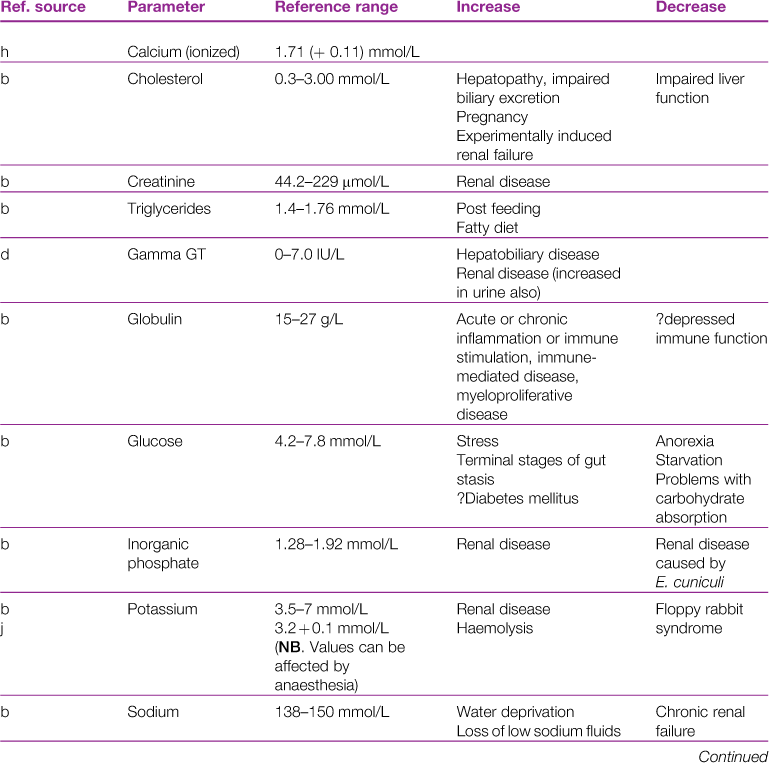
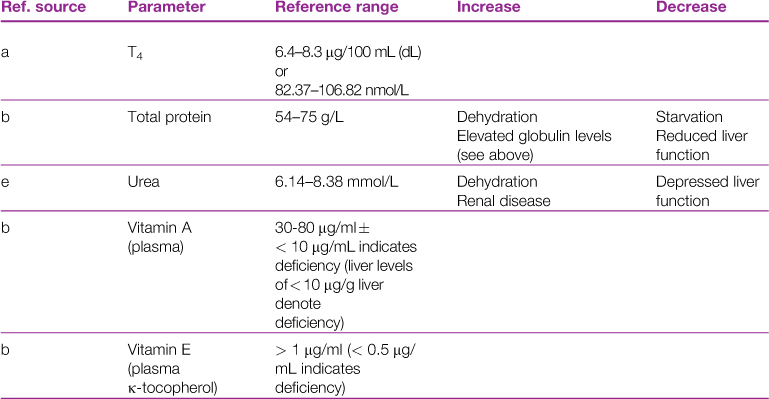
aReference sources: a, Jones (1975); b, Gillett (1994); c, Goad et al. (1989); d, Okerman (1994); e, Collins (1988); f, Swartout and Gerken (1987); g, Kerr (1989); h, Warren et al. (1989); j, Robson et al. (1981).
2.3.1 Glucose
Herbivores, such as rabbits, differ from carnivores in their carbohydrate metabolism. Carnivores eat periodically and have sudden large intakes of nutrients that must be stored for utilization during the fast between meals. Herbivores graze for long periods of the day and are continually absorbing nutrients from the digestive tract. In rabbits, volatile fatty acids are produced from bacterial fermentation in the caecum and are continually absorbed as an energy source. A fasting sample is difficult to obtain from a rabbit. Withholding food does not prevent caecotrophy and the digestion of caecotrophs provides a source of glucose. Blood samples taken after 96 h of food deprivation may show no alteration in blood glucose levels (Kozma et al., 1974).
Hyperglycaemia is a relatively common finding in rabbits and can be accompanied by glycosuria. Handling alone can cause an increase in blood glucose to the order of 8.5 mmol/L experimentally (Knudtzon, 1988) and 15 mmol/L or more anecdotally. Diabetes mellitus has not been described in pet rabbits and there is some difference of opinion about its importance as a clinical disease (Hoefer, 2000; Jenkins, 2000; Rosenthal, 2000). Herbivorous animals withstand the absence of insulin more readily than carnivorous ones (Bentley, 1998) and are therefore not so susceptible to diabetes mellitus. Diabetes mellitus has been induced in laboratory rabbits by the administration of alloxan. It has also been described as a hereditary disease in rabbits. A laboratory strain was selectively bred as an animal model of human diabetes mellitus (Roth and Conaway, 1982). Affected animals were polydipsic, polyuric and polyphagic with severely impaired insulin release. Elevated glycosylated haemoglobin values of 12.2% were observed in the overtly diabetic animals in comparison with 3.9% in normal animals. Increased glycosylated haemoglobin levels did not correlate with plasma glucose concentrations (Cannon and Conaway, 1981). Histologically there was hypergranulation of β-cells of the islets of Langerhans. Obesity and ketoacidosis were not features of diabetes mellitus in the laboratory rabbits. The hyperglycaemia was in the region of 540–590 mg/dL (30–33.4 mmol/L) and there was marked glycosuria. Roth and Conaway (1982) described the maintenance of one diabetic individual on insulin at a dose of up to 8 units per day for 3 years. Ketonuria was not observed.
In pet rabbits, a diagnosis of diabetes mellitus cannot be made on a single blood sample and requires serial blood and urine sampling to confirm the diagnosis, as well as an evaluation of fructosamine levels (performed using methodology validated for rabbits). In view of the physiological factors that can increase blood glucose levels it is advisable to take repeat blood samples from hyperglycaemic rabbits with time of day, phase of digestion, anaesthesia, influence of handling or car journeys in mind. Mild glycosuria is not a significant finding.
Hyperglycaemia can be seen in the terminal stages of gut stasis and is a poor prognostic sign (Harcourt-Brown, 2011). It is associated with fatty degeneration of the liver at post-mortem examination. Marked hyperglycaemia is also seen in association with painful conditions such as acute intestinal obstruction. Blood glucose levels can rise to 20–25 mmol/L and return to normal once the condition is resolved (Harcourt-Brown, 2011). Experimental haemorrhagic or traumatic shock results in hyperglycaemia proportional to the severity of the condition. Hyperthermia also results in hyperglycaemia (McLaughlin and Fish, 1994). Diseases that elevate serum glucose levels in other species, such as hyperadrenocorticism or acute pancreatititis, have not been reported in pet rabbits, although they could occur.
Hypoglycaemia is a significant finding in rabbits and is associated with anorexia, starvation or disturbances in the digestion and absorption of carbohydrates. It can be a sign of hepatic dysfunction. A drop in blood glucose leads to mobilization of free fatty acids from adipose tissue and contributes to the development of ketoacidosis and fatty degeneration of the liver. Measurement of serum glucose is of value in moribund rabbits as a basis for the selection of appropriate fluid therapy. Septicaemia is another potential differential diagnosis for hypoglycaemia. Other causes of hypoglycaemia such as Addison’s disease or insulinomas have not been reported in pet rabbits although such conditions could occur.
2.3.2 Total protein
Interpretation of total protein concentrations is similar to other mammals. Artefactual increases in protein concentrations can result from excessive venous stasis during blood collection (for example, when it takes a long time to get a blood sample). Fluid and small molecules leave the plasma, resulting in a relative increase in proteins. This situation can occur in rabbits, especially in miniature breeds with small veins.
An increase in total protein indicates dehydration and chronic and immune-mediated disease. In rabbits dehydration due to water deprivation or gastrointestinal disturbances commonly occur. Examination of the haematocrit and albumin and globulin fractions can assist differential diagnosis.
Liver disease, chronic enteropathy, starvation or malnutrition may result in reduced protein levels. Glomerulonephropathy or protein-losing enteropathy are uncommon conditions that could cause low total protein levels in rabbits. A decrease in both albumin and globulin may be associated with haemorrhage or exudative skin lesions such as fly strike.
2.3.3 Albumin
The liver is the sole site of albumin synthesis and hypoalbuminaemia is a feature of advanced liver disease in all species. In rabbits, heavy parasitism can be a cause of liver disease. Eimeria stiedae causes hepatic coccidiosis (see Section 8.10.1.2). Cysticercus pisiformis, the larval stage of Taenia pisiformis, migrates through the liver and results in the development of fibrous tracks and necrotic foci (see Section 14.3.2). Severe infestations can result in low albumin levels. Non-hepatic causes of low serum albumin include glomerulonephropathy, protein-losing enteropathy, malabsorption and cardiogenic ascites.
Laboratory reference ranges for serum albumin levels in rabbits can be wide and vary between sources. Sex differences have been reported in laboratory rabbits. One study showed that female New Zealand white rabbits had higher serum albumin levels than males, although other studies have found no sex differences (Kozma et al., 1974).
In rabbits, hypoalbuminaemia is most likely to be associated with nutritional factors such as abnormal caecotrophy, incorrect diet, starvation or malnutrition associated with dental disease. Primary or secondary hepatic neoplasia occasionally occurs in pet rabbits. Hepatic coccidiosis is a cause of low serum albumin levels, especially in young rabbits that have been kept in colonies.
A high serum albumin concentration is not a feature of any specific disease, although an increased albumin level in conjunction with a raised PCV is indicative of dehydration. In a study by Harcourt-Brown and Baker (2001), pet rabbits kept under free-range conditions had significantly higher serum albumin concentrations than rabbits suffering from advanced dental disease. They were also significantly higher than rabbits kept in hutches that were not suffering from dental problems. The difference in albumin levels was attributed to differences in diet and husbandry. Caecotrophs are a source of amino acids for rabbits and normal caecotrophy is an important element of their protein metabolism. Low fibre diets, obesity, dental disease or skeletal abnormalities can prevent rabbits ingesting caecotrophs from the anus and reduce the available amino acids for protein synthesis. Rabbits kept in hutches are more likely to be eating a low fibre diet and to suffer from obesity and skeletal problems than rabbits living outside with unrestricted access to natural vegetation and exercise.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree