Chapter 22
Chytridiomycosis
The emerging amphibian skin disease chytridiomycosis was first described in the late 1990s from both captive and free-ranging frogs1–3 and has rapidly become one of the most studied amphibian diseases. The causative agent, Batrachochytrium dendrobatidis (Bd), is a chytrid fungus with very low host specificity, as demonstrated by identification in more than 200 species and 20 families representing both anurans and caudates (www.bd-maps.net). Chytridiomycosis causes die-offs and population declines of free-ranging amphibians, and is a significant cause of disease and management concern in captive amphibian collections.4,5
Although commonly associated with aquatic environments, the chytrids (fungi in the Phylum Chytridiomycota) are environmentally ubiquitous, and many are involved in the degradation of plant and animal matter such as cellulose, chitin, and keratin.6,7 Before the description of Bd in amphibians, parasitic or pathogenic chytrids were recognized only in algae, fungi, invertebrate animals, and plants. Features of most chytrid fungi include a simple thallus (fungal body) in which the cytoplasm cleaves to form distinctive motile uniflagellate zoospores during asexual reproduction (Figure 22-1). Of all the chytrid fungi, only Batrachochytrium has been associated with amphibian disease, and Koch’s postulates have been fulfilled by experimental infection.8 Before the definitive descriptions of chytridiomycosis, Bd was observed by pathologists and variably reported to be a protozoan, aquatic fungal-like protist, or a clinical manifestation of infection with the fungus Basidiobolus ranarum.9–12
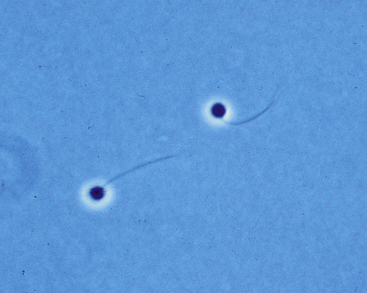
FIGURE 22-1 Zoospores of Batrachochytrium dendrobatidis as viewed by phase-contrast microscopy. The single posterior flagellum is characteristic the Phylum Chytridiomycota (×40) (Photo courtesy Dr. Joyce Longcore, The University of Maine, Orono, Me.)
Chytridiomycosis and Amphibian Population Declines
Amphibian populations worldwide are in decline, and the International Union for Conservation of Nature (IUCN) Global Amphibian Assessment has estimated that 32.5% of all amphibian species are threatened and more than 400 species may be on the brink of extinction.13 Chytridiomycosis has been identified as a major contributory factor in many of these declines.4,5,13–16 Published reports of Bd infection have come from Africa, Australia, Europe, North, Central, and South America, the Caribbean, and New Zealand.1,13,17–27 Many of the best-documented declines due to chytridiomycosis have been reported from stream-dwelling species at high elevations in Central America and Australia.28,29 In these cases, chytridiomycosis-associated declines progressed in a predictable geographic pattern suggestive of a recently introduced pathogen.1,15 In Central America, a long-term study has clearly documented loss of amphibian biodiversity only after arrival of Bd at a new location.23
There continues to be debate about whether Bd is truly an introduced novel pathogen or an endemic parasite that has increased in virulence or now causes disease secondary to environmental or other cofactors.4,16,30 Supporting the novel pathogen hypothesis are initial Bd genetic studies that showed minimal genetic variation between North American, African, and Australian Bd isolates consistent with a clonal population.31 More recent genetic studies are beginning to show more discrimination between geographically distinct isolates of Bd, including the possible emergence of a hypervirulent recombinant lineage.32–34
A leading hypothesis for international dissemination of Bd focuses on the African Clawed Frog (Xenopus laevis), which was extensively exported beginning in the 1930s for use in human pregnancy diagnosis.21 The earliest known case of Bd infection (1938) was found in the African Clawed Frog, and the prevalence of Bd infection (2.7%) in African populations has remained unchanged over many decades, which suggests endemic rather than introduced infections. In addition to the African Clawed Frog, another species that has been widely introduced worldwide and is a good potential reservoir host for Bd is the American Bullfrog (Lithobates catesbeianus).20,22,33,35 Concerns that international trade in amphibians for food, research, exhibition, teaching, and pets continues to contribute to the introduction of Bd to new areas have led to calls for national risk abatement plans and listing of Bd infection as notifiable under the Aquatic Animal Health Code of the World Organisation for Animal Health (OIE).36,37 Listing by the OIE will eventually require that amphibians moved in international trade be free of Bd infection or held in biosecure conditions to prevent introduction of Bd or virulent Bd strains to new locations.
There is great interest in the potential role of environmental cofactors in determining the outcome and significance of Bd infection in free-ranging amphibian populations. Climate-related cofactors have received the most attention because maximal growth of Bd occurs in a specific temperature range of 17°C to 25°C.38 To this end, seasonal variations with higher prevalence and severity of Bd infection in cooler times of the year (winter and spring) have been reported for some species of Australian anurans.29,39 In another article, thermal springs with water temperatures exceeding 30°C were postulated to provide refugia from Bd infection.40 The use of ecologic niche modeling has predicted that up to half of all amphibian species live in regions optimal for Bd.41 Paradoxic relationships between chytridiomycosis-associated declines and changes in microclimate associated with global warming toward those favorable for Bd have been suggested but are controversial.4,42–45 The recent report of chytridiomycosis in Andean frogs that have recently migrated to higher elevations as the result of deglaciation from environmental change represent the highest elevation and lowest temperatures from which Bd has been observed.27 Additional research is required to determine what relationships, if any, exist between the emergence of chytridiomycosis and climate change.
Interestingly, some amphibian populations that have experienced chytridiomycosis-associated declines may partially recover and persist with endemic Bd infection.40,46,47 Other amphibian populations such as those in the northeastern United States and the St. Lawrence River Valley of Quebec, Canada, show evidence of endemic Bd infection without associated declines.48,49 Because so many amphibian populations are experiencing devastating declines associated with Bd, the hope from these observations is that other populations will be able to develop similar resistance. The possibility of acquisition of resistance to Bd by either natural selection or selective captive breeding provides support for the development of captive survival assurance colonies for species affected by Bd-associated declines.50,51
Pathology and Host Defense
Infection with Bd is limited to areas of keratinizing stratified squamous epithelium in the oral disk (jaw sheaths and tooth rows) of tadpoles and the skin of postmetamorphic animals. The skin of tadpoles is nonkeratinizing and the transition to a keratinizing epidermis occurs during metamorphosis. As tadpoles undergo metamorphosis, Bd infection moves from the oral disk onto the skin with subsequent development of clinically significant disease.52–54
Invasion of Bd into deeper portions of the skin or dissemination to the visceral organs has not been observed. The primary lesion of chytridiomycosis in postmetamorphic animals is hyperkeratosis and epidermal hyperplasia (Figure 22-2). Thalli (fungal bodies) of Bd are observed in skin cell (keratinocyte) cytoplasm and are most abundant in the keratinized layers (stratum corneum) (Figure 22-3). Infiltrates of inflammatory cells or other epidermal changes such as necrosis and ulceration are inconsistently observed and are more frequently found in cases with secondary bacterial, fungal, or Oomycete (water mold) infections (see Figure 22-3). Such secondary infections are common and may occur because empty Bd thalli (those that have discharged zoospores) and the accumulated keratin layers trap environmental microorganisms.
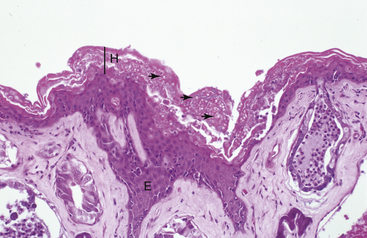
FIGURE 22-2 Lesions of severe chytridiomycosis in a White’s Tree Frog (Litoria caerulea). There is marked epidermal hyperplasia (E) and hyperkeratosis (H) with myriad thalli of Batrachochytrium dendrobatidis in the keratinized layers of the skin (arrows; ×10).
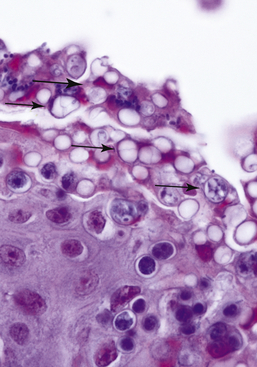
FIGURE 22-3 Chytridiomycosis in a Smoky Jungle Frog (Leptodactylus pentadactylus). There are numerous empty and developing thalli of Batrachochytrium dendrobatidis in the keratinized skin layers (arrows). In addition, there is secondary colonization of the skin with a small unidentified yeast (large arrow; ×60).
The mechanism by which chytridiomycosis causes death in susceptible animals has been thought to result from disruption of normal skin function or, alternatively, from exposure to a mycotoxin produced by Bd.1,2 Unlike other vertebrates, the skin of amphibians has important physiologic functions that include water absorption, osmoregulation, and, in some species, respiration. Studies in White’s Tree Frogs (Litoria caerulea) with experimentally induced chytridiomycosis demonstrated reduced plasma osmolality, hyponatremia, hypokalemia, hypomagnesemia, and hypochloridemia when compared with uninfected control animals.55,56 No significant differences were found in hematocrit, albumin, or urea levels, which suggests that disease interfered with mechanisms for cutaneous electrolyte transport but not with hydration status. A limited hematologic evaluation of chorus frogs (Pseudacris maculata) with naturally acquired chytridiomycosis demonstrated some evidence of hemoconcentration (suggestive of dehydration) when compared with uninfected frogs; however, results were not statistically significant and the authors felt that additional studies were required.57 At this time there is no definitive experimental evidence for the role of a mycotoxin in the pathogenesis of chytridiomycosis; however, one hypothesis for the electrolyte depletion observed in experimentally infected White’s Tree Frogs is toxin-associated disruption of cutaneous sodium channels.55 A report of rapid death in Western Toad (Anaxyrus boreas) tadpoles after inoculation with cultures of Bd was interpreted to be consistent with toxin exposure; however, a specific mycotoxin was not described.58 When Bd and chytridiomycosis were first identified, there was concern that lethal effects would be observed in all or most species that became infected. It is now known that a wide range of amphibian species—and probably most species—can become infected with Bd, however, not all species are equally susceptible to developing clinically significant chytridiomycosis, the disease caused by Bd. For instance, the Tiger Salamander (Ambystoma tigrinum), American Bullfrog, and some African Clawed Frogs become subclinically infected with Bd and may be sources of infection for other species but rarely, under both natural and experimental conditions, develop severe chytridiomycosis.20,21,35,59 Similarly, tadpoles are usually subclinically infected with Bd and are implicated as reservoirs of infection for postmetamorphic animals.1,54 Compared with animals with clinically significant chytridiomycosis, subclinically infected animals usually have minimal histologic lesions consisting of small multifocal areas of hyperkeratosis with few Bd thalli (Figure 22-4).20
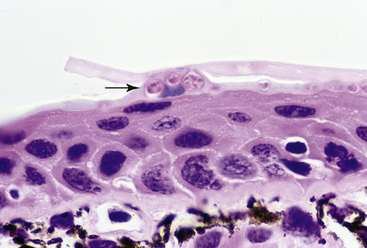
FIGURE 22-4 Subclinical infection with Batrachochytrium dendrobatidis in a Tiger Salamander (Ambystoma tigrinum). There is minimal hyperkeratosis, and superficial skin cells contain small numbers of Bd thalli (arrow; ×40).
Host factors that influence species susceptibility and the severity of chytridiomycosis in individuals may include aspects of innate and acquired immunity, differences in host skin flora, and aspects of host behavior and biology. Because Bd infection is limited to the superficial (keratinized) layers of the skin and evidence of a significant host inflammatory response is an inconsistent finding, it has been thought less likely that cellular components of innate immune response, such as phagocytic cells (neutrophils and macrophages) or adaptive immune responses (humoral or cell-mediated immunity), play a significant role in protection against Bd. However, recent studies have demonstrated evidence of specific MHC genotypes associated with increased survival of some Bd-infected animals and Bd-specific immunoglobulins in skin mucus.51,60 Not yet explored are possible roles for cytokines produced by keratinocytes or resident lymphocytes and dendritic cells that could influence keratinocyte proliferation and turnover with subsequent effects on the ability to effectively shed Bd-infected cells. Antimicrobial peptides secreted by granular glands in the skin are potentially important components of innate immunity against Bd infection.61 There are species-specific differences in the types and mixtures of peptides produced, and these differences in some instances may predict apparent resistance or susceptibility to developing severe chytridiomycosis.47,62 Complementing work on antimicrobial peptides are observations that specific symbiotic bacteria isolated from the skin of plethodontid salamanders (Plethodon cinereus and Hemidactylium scutatum) and the Mountain Yellow-legged Frog (Rana muscosa) inhibit the growth of Bd in culture and may contribute to survival of Bd-infected frogs.63,64 Finally, host behavioral factors can influence transmission of Bd and the outcome of Bd infection, and these include the frequency of frog-to-frog contact and contact with water.28,65
Nonhost factors such as environmental temperature and virulence of individual isolates (sometimes referred to as strains) of Bd may influence the severity of chytridiomycosis. Low-to-moderate environmental temperatures optimal to the growth of Bd in culture are associated with increased virulence in experimental infection studies.39,66 Furthermore, environmental temperatures higher than 27°C can result in reduced virulence and even elimination of Bd infection.39,67 A study in Panamanian Golden Frogs (Atelopus zeteki) demonstrated that thermoregulatory behavior of individual frogs influenced the likelihood of Bd infection.68 Experimental studies in Green Tree Frogs (Litoria caerulea) and Western Chorus Frogs (Pseudacris triseriata) demonstrated differences between isolates of Bd from different locations in the time to death after exposure and in the numbers of frogs killed by exposure.69,70 It has been suggested that these observations could represent variation in virulence between Bd strains. Finally, recent genetic evidence suggests that a globalized Bd lineage may be “hypervirulent” compared with other lineages.34
Clinical Signs
The spectrum of clinical signs observed in individual animals infected with Bd can vary from none in animals with low-level infections (subclinical or carrier animals) to grossly evident skin disease. Death without premonitory signs is observed in some animals with heavy infections, and the lack of grossly visible skin lesions should not be used to exclude a diagnosis of chytridiomycosis. Nonspecific clinical signs of anorexia or lethargy are common in severely affected animals. Weight loss has been observed in some experimental infection studies70; however, naturally infected animals are often in good nutritional condition. In some cases postural or behavioral changes are observed, such as reluctance to place the ventral body surface in contact with substrate or changes in (increased or decreased) desire for contact with water. Neurologic signs such as a loss of righting reflex are occasionally present.
If grossly visible skin lesions are observed, they are most often present on the ventral body and feet and include excessive skin shedding (dysecdysis), skin discoloration, and rough or granular changes in skin texture (Figure 22-5). Skin discoloration is usually brown to red and can be confused with the “redleg syndrome” (bacterial dermatosepticemia) traditionally associated with gram-negative bacteria such as Aeromonas hydrophila (Figure 22-6). Often, cases with significant cutaneous hyperemia are complicated by secondary infections. In addition to excessively shedding skin, black-pigmented spots on the ventral abdomen and dorsal head 59 or in the region around the vent were present on Tiger Salamanders and a plethodontid salamander (Plethodon neomexicanus) infected with Bd.71 In other salamanders infected with Bd, autotomization of the tail has been reported; however, the pathogenesis of this observation is uncertain.72
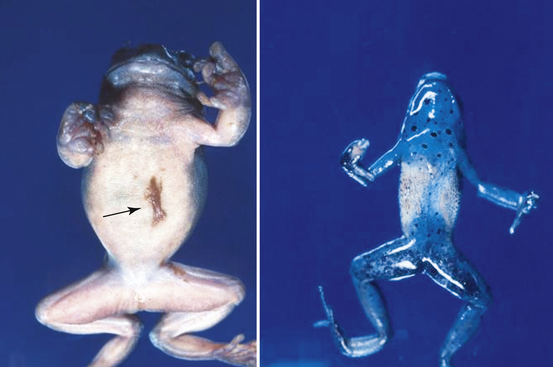
FIGURE 22-5 Gross lesions of chytridiomycosis in a White’s Tree Frog (Litoria caerulea) and Blue Poison Dart Frog (Dendrobates azureus). The White’s Tree Frog has mild hyperemia (reddening) of the ventral skin and excessive amounts of brown shedding skin (arrow). The Poison Dart Frog (right) has focally extensive areas of brown granular discoloration of the ventral skin.
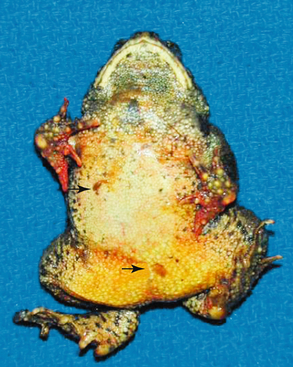
FIGURE 22-6 Gross lesions of chytridiomycosis in a Wyoming Toad (Anaxyrus [Bufo] baxteri). There are excessive amounts of brown shedding skin (arrows) and marked hyperemia (reddening) of the toes that may be confused with the ubiquitous “redleg syndrome” of captive amphibians.
Infection with Bd in tadpoles (larvae) is usually subclinical; however, grossly apparent depigmentation of portions of the oral disk have been widely reported in infected animals.54,73–75 Reduced body mass has been observed in infected tadpoles under some experimental conditions,76,77 but no evidence of decreased growth was noted in a field study of African tadpoles.78 Death was observed in Western Toad tadpoles shortly after exposure to laboratory cultures of Bd but not in tadpoles of American Bullfrogs, Cascades Frogs (Rana cascadae), or Pacific Tree Frogs (Hyla regilla).58 In free-ranging amphibian populations, chytridiomycosis has had presentations that vary from insidious, in which small numbers of deaths are observed over a period of time with gradual, but significant, loss of population numbers,79 to dramatic, with large numbers of dead animals and rapid loss of regional amphibian diversity23 (Figure 22-7). All reported chytridiomycosis-associated mortality events have affected postmetamorphic animals, and die-offs that include large numbers of larval amphibians are likely due to other infectious agents such as Ranavirus infection, even if Bd is identified in the course of the diagnostic investigation. Because the clinical signs in individual animals with chytridiomycosis may overlap with a variety of other diseases, complete investigation of all amphibian mortality events, including histopathologic examination, bacterial and virus isolation, and molecular diagnostics (e.g., polymerase chain reaction [PCR] for Bd and ranaviruses), is encouraged.
Life Cycle
The infective stage of Bd to amphibians is the motile flagellated zoospore8,66 (Figure 22-8). Zoospores are released from mature zoosporangia that have been established within amphibian keratinocytes (skin of postmetamorphic animals or oral disk of tadpoles); they then swim in the environment before reencysting. Under experimental conditions, zoospores swim very short distances (<2 cm) before encysting, and this tendency likely promotes autoinfection of host animal skin and amphibian-to-amphibian contact as mechanisms of Bd transmission.38 Dispersion of zoospores by water currents and other vectors such as bird feathers and moist substrates are also a consideration.81 Experimentally, zoospores can persist in sterilized pond water containing organic materials for as long as 7 weeks, up to 3 hours on bird feathers, and at least 12 weeks in sterilized creek bed sand.80,81 A report of freshwater shrimp as a possible reservoir host for Bd was later retracted.82 Determination of the importance of reservoirs for Bd outside of an amphibian host is the subject of active ongoing research and will be aided by PCR techniques that detect Bd in environmental samples.83,84 The use of mathematical models has suggested that the ability of Bd to persist outside of an amphibian host may increase the probability of driving host animals toward extinction.85 Unlike some other chytrid fungi, a resting spore that could be involved in long-term environmental persistence or long distance dispersal has not been identified for Bd. However, the recent observation of probable sexual reproduction raises the possibility that a resting spore may occur under some circumstances.32 Approaches to the identification of subclinically infected amphibians that may serve as reservoir hosts are discussed in the sections on Morphologic Methods of Diagnosis and Molecular Methods of Diagnosis.
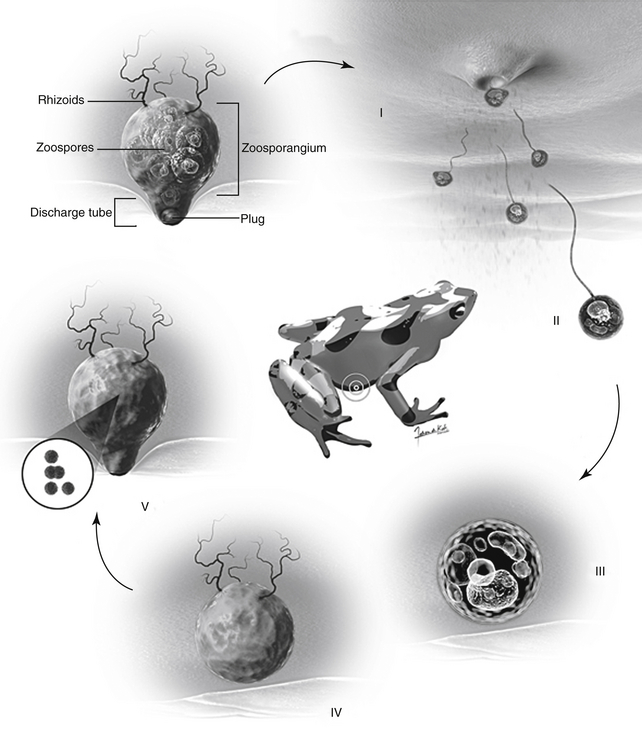
FIGURE 22-8 The life cycle of Batrachochytrium dendrobatidis (Bd) in an amphibian host. I, A mature thallus (zoosporangium) of Bd inside an amphibian skin cell (keratinocyte) releases uniflagellate zoospores through a discharge tube oriented toward the environment. II, Zoospores swim a short distance or may be carried by water currents. III and IV, The zoospore encounters a new amphibian keratinocyte on the same or different animal and begins to develop a new thallus within the cell cytoplasm. V, The cytoplasm of the thallus cleaves to form discrete zoospores culminating in a new zoosporangium to begin the cycle anew (Courtesy Fabian de Kok-Mercado, CMI, Johns Hopkins University, Baltimore, Md.)
Diagnosis and Treatment
Transmission and Disinfection
In captive situations it has been possible to house Bd-infected animals adjacent to noninfected animals as long as special care is taken to avoid transfer of animals, water, substrates, or cage decorations (including plants) between enclosures.8,86 Protocols that include disinfection of instruments and wearing of gloves (or hand washing) between servicing different enclosures should be incorporated into the standard operating procedures of amphibian collections. Most of the common disinfectants used in captive collections and veterinary hospitals, as well as heat and complete drying (desiccation), have been shown experimentally to kill Bd38,86, 87-89 (Table 22-1). Ultraviolet light has been shown to be ineffective against cultures of Bd in the laboratory.87 Before disinfection, enclosures and instruments should always be thoroughly cleaned to remove organic materials that will inactivate most chemical disinfectants. Disinfectant concentrations and application times beyond the minimum suggested by the experimental data are given. In addition to disinfection of equipment and enclosures, consideration should be given to treatment of waste water and substrates used in amphibian collections to minimize the risk of introducing Bd (or other pathogens) to free-ranging amphibian populations.
TABLE 22-1
Chemical and Physical Methods for Elimination of Batrachochytrium dendrobatidis on Surfaces
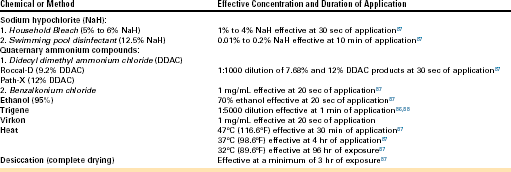
Heat and complete drying of surfaces may be most useful in real-world settings when used together or in combination with a chemical disinfection method. For instance, enclosures can be thoroughly cleaned with hot water, disinfected with an appropriate chemical, rinsed thoroughly to eliminate chemical residues, and then set out to dry in a warm place or room (at least 4 hours at 37°C or 98.6°F). Heating of wastewater, wet substrates, and plants before disposal (or before use of heat tolerant plants or substrates that may have been exposed to potentially infected amphibians) is a potentially environmentally friendly approach to disinfection of these items.
Disinfection of items used in amphibian field research programs is an important approach to limiting movement of Bd (or other amphibian pathogens not yet identified) from a location with infected animals to a naïve location that has not yet encountered this organism. Suggested protocols for disinfection of field equipment are available online at the Amphibian Diseases Homepage (http://www.jcu.edu.au/school/phtm/PHTM/frogs/ampdis.htm). Similar to enclosures or equipment used in captive management of amphibians, field equipment should be thoroughly cleaned of dirt or other organic material before disinfection. It has been suggested that at least twice the experimentally effective concentration of disinfectant should be used.88
Diagnosis
A variety of morphologic and molecular techniques have been described to diagnose chytridiomycosis or to detect Bd infection in amphibians. The choice of a diagnostic method depends on factors that include the purpose of the examination, the type of sample available for analysis, and access to specific equipment or reference laboratories.89 For diagnostic testing, it is important to distinguish between clinical disease or death due to Bd infection (chytridiomycosis) and subclinical infection with Bd. In general, morphologic methods such as wet mounts, cytology, and histopathology are most useful for diagnosing clinically significant Bd infections that have high numbers of organisms present in the skin. In contrast, molecular methods (conventional or Taqman PCR) are considerably more sensitive and have clinical applications that include confirming clinical diagnoses, detecting subclinical infections in quarantine, and performing surveys to detect the presence or prevalence of Bd infection in captive or wild populations.90,91 Routine fungal culture is not useful for diagnosis because Bd requires specific isolation techniques and media3,38 (Figure 22-9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree