Chapter 53 Chronic Wasting Disease of Cervid Species
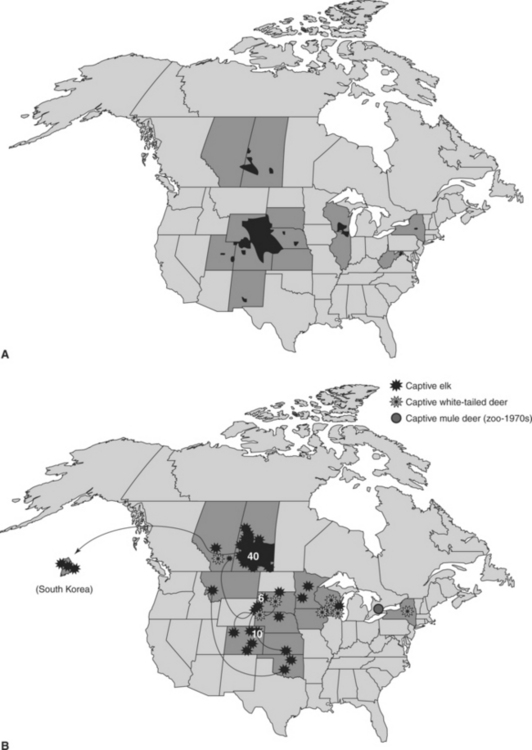
Chronic wasting disease (CWD), a contagious prion disease of several cervid species, has emerged from obscurity to become what some consider one of the most important wildlife health problems in North America. Although the long-term implications and relative importance of CWD remain to be determined, the detection and apparent extent of CWD in both free-ranging and captive cervid populations already have had appreciable impacts on both wildlife management and the captive cervid industry44–46 (Figure 53-1).
ETIOLOGY
Chronic wasting disease is one of a group of unconventional diseases originally termed “transmissible spongiform encephalopathies” (TSEs) and is one of three TSEs that occur in domestic ruminants in North America; the other two are scrapie of sheep and goats and bovine spongiform encephalopathy (BSE) of cattle. The TSEs appear to be caused by proteinaceous infectious agents (prions),33 although a viral or virinal etiology has not been completely disproved. As with other prion diseases, the CWD agent (prion or otherwise) has not been isolated or fully characterized. A single strain of CWD prion has been recognized thus far, but data from several studies suggest the possibility of CWD strain variation.3,34,35 The relatively wide natural host range (four species in three different genera) and polymorphisms in the prion gene of each host species provide a plausible biologic mechanism for such variation in cervid-associated prion strains to arise.
The origin of CWD is not known, but three possible sources have been hypothesized.44,45,50 First, CWD may be a new or a reemerging disease of cervids; its relatively limited global distribution and poor infectivity in bovids support this hypothesis. Second, CWD may be the result of scrapie infection in cervids; the many similarities to scrapie pathogenesis and epidemiology and lack of cohesive epidemiologic explanations for all the CWD foci detected in North America support this hypothesis. The third, more remote possibility is that CWD arose from an as-yet-unrecognized prion strain originating in another domestic or free-ranging species. The relationship between CWD and scrapie and the potential for scrapie exposure to initiate new CWD foci in North America and elsewhere have not been defined. Although some uncertainty exists about the relationship between CWD and scrapie prion strains, laboratory and epidemiologic data have shown more clearly that the CWD strain is different from the prion strain that causes BSE.4,34,43
EPIDEMIOLOGY
Chronic wasting disease occurs naturally in four species in three genera, all North American members of the family Cervidae. Infections in mule deer (Odocoileus hemionus) and white-tailed deer (O. virginianus) are more common than in wapiti (Cervus elaphus nelsoni)28,45; a single natural case has been reported recently in moose (Alces alces).20 There appear to be molecular barriers limiting the potential natural host range of CWD.35 The notion of a limited natural host range for CWD is consistent with its absence in other free-ranging or domestic North American ruminant species with opportunities for natural exposure.10,46 As with other prion diseases, however, CWD has been transmitted to a variety of species by experimental intracerebral inoculation.44,45,50
Chronic wasting disease is contagious among susceptible species, but knowledge about the exact mechanism(s) of natural transmission remains incomplete. Shedding of agent in feces with fecal-oral transmission seems the most plausible route of CWD transmission, but other possible mechanisms cannot be discounted.23,26,38,45,49 Regardless of the precise mechanism, direct or indirect horizontal transmission apparently sustains epidemics. Maternal transmission, if it occurs, is of limited importance.26 Live, infected animals likely are the main sources of new infections and geographic spread.* Environments contaminated with excreta or decomposed carcasses may harbor some infectivity for years.23 No evidence has emerged suggesting that CWD is associated with a food-borne pathogen, as is the case with BSE. Deer and wapiti held in affected game farms have not been shown to have consumed rendered ruminant proteins. Similarly, free-ranging deer and wapiti feed on natural forage and have not had known contact with rendered feeds or byproducts in places where CWD epidemics have occurred.
Genetics appear to influence CWD susceptibility and pathogenesis.44 For the three main natural host species, one or more substitution polymorphisms have been identified in the protein-coding region of the native prion gene in wapiti at codon 132 (methionine [M] or leucine [L]),30 in mule deer at codons 20 (aspartate [D] or glycine [G], removed during processing) and 225 (serine [S] or phenylalanine [F]),2 and in white-tailed deer at codons 95 (glutamine [Q] or histidine [H]), 96 (G or S), and 116 (alanine [A] or G).31 Deer and wapiti of all recognized prion protein (PrP) genotypes may be infected with CWD; however, individuals of the less common genotypes in each host species tend to be underrepresented among infected conspecifics, and the onset of clinical disease in such individuals may be delayed.12,15,16,30,31
Cases of CWD may occur at any time of year. In the wild, however, these tend to be observed more often in the fall and winter28,41; a similar pattern also occurs in captive cervids. Among mule deer, infection rates are higher in males and older individuals, suggesting differential exposure risks between genders and across age classes22,28; similar patterns would be expected in the other host species.
Although surveillance for CWD began in some areas in the early 1980s, most of the effort directed toward determining its distribution and prevalence in free-ranging and captive cervids in North America and elsewhere has occurred since 2000.28,41,45,47,50 Detecting CWD foci in free-ranging populations is challenging because disease distribution appears to be patchy and sampling is rarely uniform.28,37 Overall prevalence of CWD infection in endemic areas may reach 1% to 5% or higher in free-ranging populations.17,28 The perception that CWD has “spread” dramatically since 2000 seems more likely an artifact of the increase in surveillance activities undertaken in recent years rather than a true expansion in the number of new foci over this period.
Under captive conditions, CWD prevalence may be even higher than observed among cervids in the wild. In captive mule deer and white-tailed deer, entire cohorts have become infected and succumbed to CWD over the course of several years.23,26,48 High prevalence also has been reported in captive wapiti, but this pattern is not as consistent as seen in captive deer.18,24,32,49 Factors contributing to the remarkably high prevalence sometimes seen in captive deer and wapiti remain to be determined, although intensive environmental contamination and repeated exposure to infectious materials seem likely contributors.
CLINICAL SIGNS AND COURSE
The image of a drooling, stumbling, emaciated deer or wapiti has been represented and repeated so often as the “classic” presentation for clinical CWD over the years that many clinicians, wildlife managers, and cervid caretakers might not recognize a more typical clinical case if presented with one (Figure 53-2, A). It follows that failure to recognize and properly investigate suspect CWD cases may be partially responsible for delayed detection of foci in both captive and free-ranging cervids and for gaps in epidemiologic investigations of its occurrence and spread.
Affected animals eventually lose weight even though they continue to eat, but weight loss may be episodic.24 In individuals that survive to the terminal stages of CWD, body condition will decline appreciably and irreversibly. Clinical signs tend to be more difficult to recognize and more rarely reported in free-ranging cervids, perhaps because they may mimic normal seasonal variation in behavior and condition, and because obviously affected individuals may not survive long enough in the wild to show signs consistently (Figure 53-2, B).
In all cases to date, affected animals did not recover from clinical CWD.44,45,50 As with its signs, the clinical course of CWD is variable and may last from a few weeks to perhaps a year or more. In part, the apparent duration of clinical disease depends on the astuteness of observers in recognizing subtle early clinical signs. In deer the clinical course is sometimes very short, lasting only a few days, and infected animals have died acutely without displaying typical clinical signs.23 “Sudden deaths” following handling also have been reported in some situations, as have unusual mortalities (e.g., an animal getting its head caught under a feed trough or entangled in a low fence). The PrP genotype also may influence the overall length of the disease course in both deer and wapiti.12,15
Clinical CWD in both deer and wapiti is preceded by a relatively long incubation period. Little is known about the average or maximum length of the incubation period for natural infections, but because end-stage clinical CWD has been reported in deer and wapiti less than 2 years of age, a minimum of 1 to 1.5 years seems likely. Such estimates are consistent with observations of incubation periods after oral experimental infections.44,45 Clinical CWD has been manifested in individuals as old as 15 years, but in such cases of older onset, the timing of exposure has not been determined.45,50 The onset and severity of clinical CWD appear to coincide generally with the timing and progression of abnormal prion accumulation and spongiform degeneration in the brain and spinal cord of infected individuals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree