CHAPTER 36 Chronic Disease Management
Immunosuppressive Drug Therapy
Glucocorticoids
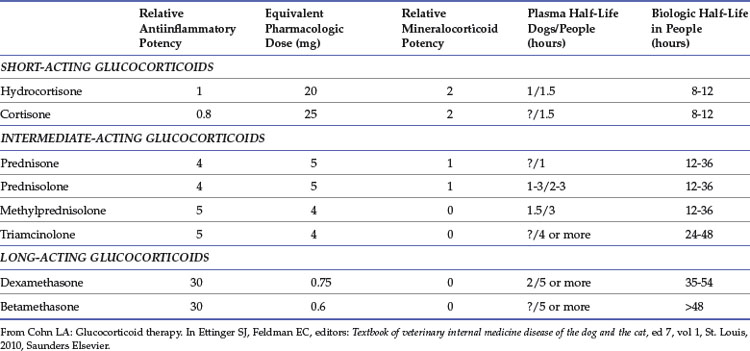
Glucocorticoids are by far the most commonly used immunosuppressive drugs. They affect nearly every tissue in the body to alter metabolism and suppress inflammation and immune responses in a dose-dependent fashion. Genomic transcription patterns and subsequent protein expression are altered, resulting in impaired cell-mediated immunity, and to a lesser extent, impaired phagocytic and humoral immunity.8 A multitude of GCs are available that vary in potency and route of administration. Based on data largely derived from other species, these drugs are described as short-, intermediate-, or long-acting based on duration of suppression of the hypothalamic-pituitary-adrenal axis (HPAA; Table 36-1). Duration of action depends upon both the base compound and modifications, such as esterification, that alter GC absorption. For instance, although methylprednisolone is an intermediate-acting compound, it is a very long-acting compound as the repositol formulation methylprednisolone acetate. When used to achieve chronic immunosuppression, intermediate-acting preparations, such as prednisolone, offer the advantage of a practical dose regimen, which allows the dose to be tailored to produce efficacy while minimizing HPAA suppression and adverse effects. The most commonly prescribed GCs are prednisone or its active metabolite prednisolone. In cats, prednisolone is strongly preferred versus prednisone, because of a superior pharmacokinetic profile.12 The mechanism behind the disparity is not entirely clear, but decreased gastrointestinal (GI) absorption of prednisone or diminished hepatic conversion of prednisone to prednisolone are suspected.
Surprisingly, there is no scientific evidence of exactly what constitutes an immunosuppressive dose of prednisolone (or any other GC) in cats. In dogs, 2 to 4 mg/kg/day has been accepted as an initial immunosuppressive dose. Recommendations for initial immunosuppression in cats range from 2 to 8 mg/kg, while antiinflammatory dosages range from 0.5 to 2 mg/kg/day of prednisolone. Compared with dogs, cats are relatively resistant to many of the effects of GC, perhaps because of lesser numbers of intracytoplasmic GC receptors.37 However, in the authors’ opinion, dosages greater than 4 mg/kg/day are not required; higher dosages recommended in years past may have been based on clinical experience using less biologically available prednisone instead of prednisolone. The initial dose is continued for several days past clinical remission and then gradually tapered over months. In general, the dose may be reduced approximately 25% every 3 to 4 weeks as long as disease remains controlled. In some cases, GCs are continued at the lowest effective dose for an indefinite (even lifelong) period. Disease control should be monitored during dose reduction, a task more easily accomplished for diseases in which there is a measurable end point (e.g., hematocrit in cats with immune-mediated anemia). Although it is tempting to reduce dosages rapidly, early withdrawal may predispose to relapse. Biologically equivalent dosages of other GCs can be used in place of prednisolone (e.g., dexamethasone at 0.3 to 0.55 mg/kg/day). Long-acting repositol formulations of GCs (e.g., methylprednisolone acetate) can be used when owners are unable to administer daily oral medications, but fine adjustments become impossible and adverse effects may be more likely.
Adverse reactions of GCs are usually associated with high-dose regimens, and extended administration protocols, such as those used in chronic immune suppression. Although cats are relatively resistant to GC, adverse effects can develop. Polyuria, polydipsia, and polyphagia are less common in cats than in dogs but do occur, while a variety of other adverse effects of GCs have been documented or suggested (e.g., alopecia, pancreatitis).8 In addition to a stress leukogram, hyperglycemia, hyperalbuminemia, and hyperlipidemia were noted in healthy cats given prednisolone (4.4 mg/kg/day) or dexamethasone (0.55 mg/kg/day) for 56 days.18 These same cats developed hepatic glycogen deposition as well.18 The metabolic actions of GCs on glucose balance result in hyperglycemia in healthy cats, and some cats treated with GC develop either temporary or permanent diabetes mellitus.19,22 The diabetogenic effects of dexamethasone may be more pronounced than those of prednisolone.18,19 The use of high-dose, long-term systemic GC should be avoided whenever possible in diabetic cats. Cardiac disease and especially congestive heart failure are also relative contraindications to GC use, since GC-associated water retention may exacerbate congestive failure.35 As with any immunosuppressive therapy, an additional relative contraindication is infection.
Adverse effects of GCs can be minimized by limiting systemic exposure. In addition to using the lowest effective dose of an intermediate-acting GC, this can be achieved by local application of GC whenever possible. In some cases, GCs are formulated in such a way that systemic absorption is limited after local application, and/or absorbed GC is rapidly inactivated through first-pass hepatic metabolism. For example, application of the GCs fluticasone or flunisolide by nebulization or metered dose inhaler to the airway epithelium of cats with feline reactive airway disease (e.g., asthma) delivers the drug directly to the disease site, preserving efficacy while limiting systemic GC exposure.9,28,29 Similarly, oral budesonide has been used to treat inflammatory bowel disease in dogs, because it delivers GC to the GI epithelium but limits systemic exposure as a result of limited intestinal absorption and first-pass hepatic metabolism.36 There are no studies showing efficacy of this agent in cats; anecdotal reports suggest variability in clinical response. When adverse effects of GC are pronounced or when GC alone fails to control disease, alternative immunosuppressive drugs may allow a decrease in GC dose or may even permit discontinuation of GC therapy.
Immunophilin Ligands
The most commonly used immunosuppressive drug in this class is cyclosporine. Cyclosporine inhibits T-lymphocyte activation and inhibits synthesis of cytokines, such as interleukin-2 (IL-2) and gamma interferon, while also reducing activation of antigen-presenting cells and phagocytes.13,30 Used extensively to prevent rejection of transplanted kidneys, cyclosporine has also been used to treat immune-mediated blood disorders in cats, including immune hemolytic anemia, pure red cell and megakaryocytic aplasia, and immune thrombocytopenia.6,15,33,34 Cyclosporine has also been used successfully to treat a variety of feline skin diseases, including atopic dermatitis, feline eosinophilic granuloma complex, feline urticaria pigmentosa, pemphigus erythematosus, feline pruritus, atopic dermatitis, granulomatous folliculitis, and furunculosis as well as sebaceous adenitis.24,25,38,40 Anecdotally, cyclosporine has been used to treat inflammatory bowel disease as well.1 Cyclosporine has been explored as an additional therapy for feline asthma, with conflicting results. One experimental study suggested cyclosporine inhibited airway remodeling and inflammation, while a second study found no change in initial asthmatic response or mast cell degranulation of experimentally sensitized animals.23,26
Cyclosporine is poorly soluble in water. Microemulsified formulations (e.g., Neoral, Atopica) are preferred rather than the original nonaqueous suspension. An initial dose of 5 mg/kg, PO, every 12 hours is reasonable, but there is enough individual variation in absorption that dosage must be adjusted based upon measured drug concentration.21Fortunately, the small size of cats makes the cost of treatment reasonable.
Compared with other immunosuppressive therapies, cats tolerate cyclosporine well. Unlike cytostatic drugs, cyclosporine is not myelosuppressive. The most common adverse effect associated with cyclosporine is GI irritation. Hepatotoxicosis or nephrotoxicosis, although rare, is more serious.24,30 Because there are multiple case reports of cats developing systemic toxoplasmosis during treatment with cyclosporine, the authors recommend that toxoplasma titers be determined prior to initiation of therapy.2,5,17 Risk must be weighed against benefit for any cat with positive titers. Cats treated with cyclosporine should be monitored with a complete blood count (CBC) and serum chemistry profile at least 3 times per year. In a group of feline renal transplant patients treated with cyclosporine and prednisolone, malignant neoplasias occurred at more than 6 times the expected rate.32
Tacrolimus is an immunophilin ligand immunosuppressive agent that is available in both oral and topical formulations, and it has a mechanism of action similar to cyclosporine. It is used in humans to prevent graft rejection and has also been evaluated for prevention of renal allograft rejection in cats.16 Tacrolimus provided marked improvement in the a small series of cats with proliferative and necrotizing otitis externa refractory to GC, antibiotic, and antifungal therapy.20
Cytostatic Drugs
Alkylating agents act by causing cross-linkage and strand breaks in DNA and RNA. They are commonly used as chemotherapeutic drugs, but they also act on lymphocyte populations impairing both cell-mediated and humoral immunity. Because these agents require at least several weeks to become effective, they are begun along with GC and continued after the GC is tapered or discontinued. The two alkylating agents used most often as immunosuppressant therapies in cats are chlorambucil and cyclophosphamide. Both have been used as adjunctive or alternative therapies to GC in cats with inflammatory bowel disease (IBD).1 Occasionally, alkylating agents have been used to treat hematologic disorders as well.34
Many feline practitioners prefer chlorambucil to cyclophosphamide, because it seems to be better tolerated, but there is little to document an advantage of one versus the other. Chlorambucil is available in a 2-mg tablet form convenient for use in cats. Most cats can be given 2 mg, PO, every 48 hours initially. The dose frequency may be adjusted from every 24 to every 96 hours, depending on the response of the cat to treatment. Both chlorambucil and cyclophosphamide can induce myelosuppression; so, a CBC must be monitored on a regular basis. Initially a CBC should be checked 7 to 10 days after beginning therapy, and even during chronic therapy, a CBC should be monitored at least every 60 days. Additional adverse effects include GI upset and myoclonus (muscle twitching) for chlorambucil or hemorrhagic cystitis for cyclophosphamide.4,10
Cytostatic antimetabolite drugs mimic molecules that participate in cellular biochemical reactions but differ enough from the natural molecule to interfere with normal cell division and function. They include nucleic acid analogues as well as antifolate drugs. Most have a more profound effect on T lymphocytes (and therefore on cell-mediated immunity) than on B-lymphocytes. Azathioprine is an antimetabolite commonly used to induce and maintain immunosuppression in dogs and humans. The drug is metabolized to 6-mercaptopurine (6-MP), which interferes with de novo purine synthesis. Unfortunately, a profound and potentially fatal myelosuppression occurs more commonly in cats treated with azathioprine than in dogs or humans, preventing its routine use in felines.3,27 This difference in the response of cats compared with other species is likely the result of a relative deficiency in the enzyme that catalyzes the conversion of 6-MP to inactive metabolites.11,31
Methotrexate is an antimetabolite used to treat rheumatoid arthritis in humans and is occasionally used for chemotherapy or as an immunosuppressive drug in cats. The drug has been used in combination with another antimetabolite drug, leflunomide (Arava), in a small number of cats with spontaneous erosive rheumatoid arthritis.14 Leflunomide has been used to treat dogs with a wide variety of immune-mediated diseases, but experience in cats is more limited. Leflunomide is converted to an active metabolite that inhibits an enzyme crucial for de novo pyrimidine synthesis. There has been some interest in the use of leflunomide for immunosuppression in feline renal transplantation, since the drug also possesses antiherpesvirus activity.39
Antibodies
Antibodies can be used to cause therapeutic immunomodulation. For instance, humanized murine monoclonal antibodies directed against the CD3 molecule on T-lymphocyte receptors are quite effective in the prevention of organ rejection in people. However, human or humanized antibodies may not be effective or safe in cats. To the author’s knowledge, the single drug in this class that has been used in feline medicine is human intravenous immunoglobulin (IV-Ig). Derived from a pooled human donor population, IV-Ig contains human polyvalent antibody consisting of predominantly IgG antibodies. Originally developed to treat antibody deficiency syndromes, it has become well accepted for the acute treatment of immune-mediated disease in humans. Although the mechanisms of action are poorly understood, competitive blockade of Fc receptors on macrophages, inhibition of complement activity, and alterations in T-lymphocyte and B-lymphocyte function may each play a role.7 In cats, IV-Ig has been used to treat severe erythema multiforme and immune-mediated erythroid and megakaryocytic aplasia with a good outcome.7,41 Although adverse effects were not reported in the few published case reports, it is reasonable to assume that a human-derived protein may lead to anaphylactic reactions, especially with repeated use. Although IV-Ig may eventually be shown to have some utility in initial stabilization of cats with life-threatening immune-mediated disease, this expensive therapy is unlikely to have a role in chronic immunosuppression.
1 Allen H. Therapeutic approach to cats with chronic diarrhea. In: August J, editor. Consultations in feline internal medicine. ed 6. St Louis: Elsevier; 2010:244.
2 Barrs VR, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporin therapy. Aust Vet J. 2006;84:30.
3 Beale KM, Altman D, Clemmons RR, et al. Systemic toxicosis associated with azathioprine administration in domestic cats. Am J Vet Res. 1992;53:1236.
4 Benitah N, de Lorimier LP, Gaspar M, et al. Chlorambucil-induced myoclonus in a cat with lymphoma. J Am Anim Hosp Assoc. 2003;39:283.
5 Bernsteen L, Gregory CR, Aronson LR, et al. Acute toxoplasmosis following renal transplantation in three cats and a dog. J Am Vet Med Assoc. 1999;215:1123.
6 Bianco D, Armstrong PJ, Washabau RJ. Presumed primary immune-mediated thrombocytopenia in four cats. J Feline Med Surg. 2008;10:495.
7 Byrne KP, Giger U. Use of human immunoglobulin for treatment of severe erythema multiforme in a cat. J Am Vet Med Assoc. 2002;220:197.
8 Cohn LA. Glucocorticoid therapy. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine: diseases of the dog and the cat. ed 7. St Louis: Elsevier; 2010:503.
9 Cohn LA, Declue AE, Cohen RL, et al. Effects of fluticasone propionate dosage in an experimental model of feline asthma. J Feline Med Surg. 2009;12:91.
10 Crow SE, Theilen GH, Madewell BR, et al. Cyclophosphamide-induced cystitis in the dog and cat. J Am Vet Medical Assoc. 1977;171:259.
11 Foster AP, Shaw SE, Duley JA, et al. Demonstration of thiopurine methyltransferase activity in the erythrocytes of cats. J Vet Intern Med. 2000;14:552.
12 Graham-Mize CA, Rosser EJ, Hauptman J. Absorption, bioavailability and activity of prednisone and prednisolone in cats. In: Hillier A, Foster A, Bertola G, et al, editors. Advances in veterinary dermatology. ed 5. Ames, Iowa: Blackwell Publishing; 2005:152.
13 Guaguere E, Steffan J, Olivry T. Cyclosporin A: a new drug in the field of canine dermatology. Vet Dermatol. 2004;15:61.
14 Hanna FY. Disease modifying treatment for feline rheumatoid arthritis. Vet Comp Orthop Traumatol. 2005;18:94.
15 Husbands B, Smith SA, Weiss DJ. Idiopathic immune mediated hemolytic anemia (IMHA) in 25 cats [abstract]. J Vet Intern Med. 2002;16:350.
16 Kyles AE, Gregory CR, Craigmill AL, et al. Pharmacokinetics of tacrolimus after multidose oral administration and efficacy in the prevention of allograft rejection in cats with renal transplants. Am J Vet Res. 2003;64:926.
17 Last RD, Suzuki Y, Manning T, et al. A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet Dermatol. 2004;15:194.
18 Lowe AD, Campbell KL, Barger A, et al. Clinical, clinicopathological and histological changes observed in 14 cats treated with glucocorticoids. Vet Rec. 2008;162:777.
19 Lowe AD, Graves TK, Campbell KL, et al. A pilot study comparing the diabetogenic effects of dexamethasone and prednisolone in cats. J Am Anim Hosp Assoc. 2009;45:215.
20 Mauldin EA, Ness TA, Goldschmidt MH. Proliferative and necrotizing otitis externa in four cats. Vet Dermatol. 2007;18:370.
21 Mehl ML, Kyles AE, Craigmill AL, et al. Disposition of cyclosporine after intravenous and multi-dose oral administration in cats. J Vet Pharmacol Ther. 2003;26:349.
22 Middleton DJ, Watson AD. Glucose intolerance in cats given short-term therapies of prednisolone and megestrol acetate. Am J Vet Res. 1985;46:2623.
23 Mitchell RW, Cozzi P, Ndukwu IM, et al. Differential effects of cyclosporine A after acute antigen challenge in sensitized cats in vivo and ex vivo. Br J Pharmacol. 1998;123:1198.
24 Noli C, Scarampella F. Prospective open pilot study on the use of ciclosporin for feline allergic skin disease. J Small Anim Pract. 2006;47:434.
25 Noli C, Toma S. Three cases of immune-mediated adnexal skin disease treated with cyclosporin. Vet Dermatol. 2006;17:85.
26 Padrid PA, Cozzi P, Leff AR. Cyclosporine A inhibits airway reactivity and remodeling after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1996;154:1812.
27 Paul AL, Shaw SP, Bandt C. Aplastic anemia in two kittens following a prescription error. J Am Anim Hosp Assoc. 2008;44:25.
28 Reinero CR, Brownlee L, Decile KC, et al. Inhaled flunisolide suppresses the hypothalamic-pituitary-adrenocortical axis, but has minimal systemic immune effects in healthy cats. J Vet Intern Med. 2006;20:57.
29 Reinero CR, Decile KC, Byerly JR, et al. Effects of drug treatment on inflammation and hyperreactivity of airways and on immune variables in cats with experimentally induced asthma. Am J Vet Res. 2005;66:1121.
30 Robson D. Review of the pharmacokinetics, interactions and adverse reactions of cyclosporine in people, dogs and cats. Vet Rec. 2003;152:739.
31 Rodriguez DB, Mackin A, Easley R, et al. Relationship between red blood cell thiopurine methyltransferase activity and myelotoxicity in dogs receiving azathioprine. J Vet Intern Med. 2004;18:339.
32 Schmiedt CW, Grimes JA, Holzman G, et al. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immunosuppression. Vet Comp Oncol. 2009;7:45.
33 Schmiedt CW, Holzman G, Schwarz T, et al. Survival, complications, and analysis of risk factors after renal transplantation in cats. Vet Surg. 2008;37:683.
34 Stokol T, Blue JT. Pure red cell aplasia in cats: 9 cases (1989-1997). J Am Vet Med Assoc. 1999;214:75.
35 Trasida P, Tobias AH, Smith SA, et al. Hemodynamic effects of methylprednisolone acetate administration in cats. Am J Vet Res. 2006;67:583.
36 Tumulty JW, Broussard JD, Steiner JM, et al. Clinical effects of short-term oral budesonide on the hypothalamic-pituitary-adrenal axis in dogs with inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40:120.
37 Van den Broek AH, Stafford WL. Epidermal and hepatic glucocorticoid receptors in cats and dogs. Res Vet Sci. 1992;52:312.
38 Vercelli A, Raviri G, Cornegliani L. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol. 2006;17:201.
39 Williams CR, Sykes JE, Mehl M, et al. In vitro effects of the active metabolite of leflunomide, A77 1726, on feline herpesvirus-1. Am J Vet Res. 2007;68:1010.
40 Wisselink MA, Willemse T. The efficacy of cyclosporine A in cats with presumed atopic dermatitis: a double blind, randomised prednisolone-controlled study. Vet J. 2009;180:55.
41 Zini E, Hauser B, Meli ML, et al. Immune-mediated erythroid and megakaryocytic aplasia in a cat. J Am Vet Med Assoc. 2007;230:1024.
Monitoring Long-Term Therapy
Clinical Monitoring
1 Educating both oneself and the client about adverse effects and indicators of efficacy associated with a particular drug
2 Making follow-up calls to detect adverse effects, since clients will not necessarily report this information without prompting
3 Accurately recording physical examination findings at each recheck visit
4 Having clients keep a treatment log in order to note changes in their pets over time
Monitoring issues associated with some feline drugs for which efficacy assessment is primarily based on clinical observation are listed in Table 36-2.
TABLE 36-2 Summary of Monitoring Recommendations for Long-Term Drugs That Have Primarily Clinical Efficacy Indices in the Cat*
| Drug or Drug Class | Potential Adverse Effects | Monitoring Recommendations |
|---|---|---|
| Antifungals (G = griseofulvin, I = itraconazole, T = terbinafine) | Bone marrow suppression; hepatotoxicity; ataxia (G)18,26,42; dose-dependent anorexia and vomiting (I)27; increased ALT(I)57,58; alter therapy if severe or symptomatic; rare hepatotoxicity in humans (T)11 | CBC +/− liver enzymes before and every 1 to 4 weeks during therapy (G); liver enzymes every 2 to 4 weeks (I); consider baseline and periodic liver enzyme assessment (T) |
| Antihistamines (e.g., chlorpheniramine) | Transient sedation31 | Data limited; safety monitoring is primarily clinical at this time |
| Antivirals | Anemia with zidovudine (AZT) in cats with FeLV17and FIV16; mild diarrhea and fatigue with feline IFN omega10; transient anorexia and weight loss with high-dose human IFN alpha59 | PCV periodically (AZT); if <20%, discontinue temporarily and restart at lower dose16 |
| Appetite stimulants (mirtazapine, cyproheptadine) | Anecdotally, hyperexcitability and vocalization with mirtazapine (possible serotonin syndrome)1,40; may respond to dose reduction or treatment with cyproheptadine28,56; vomiting, vocalization, and sedation with cyproheptadine (infrequent)47 | Data limited; safety monitoring is primarily clinical at this time |
| Cobalamin | None documented in cats; clinical effect measured by improved appetite, weight gain, decreased GI signs43 | Serum levels can be measured; however, dosing is usually empirical |
| Fluoroquinolones (long-term use not ideal) | Retinal toxicity at doses as low as 4.6 mg/kg/day (see Chapter 29 for further details) | Monitor for mydriasis or vision loss; discontinue drug if noted |
| Metronidazole | Neurotoxicosis at chronic doses >58 mg/kg/day3,33; reversible DNA disruption after 7 days of treatment (clinical significance unclear)49 | Monitor for neurologic abnormalities; consider alternative medications for chronic therapy |
| Meloxicam (chronic use off label in some countries) | Renal and GI toxicity class effect; discontinue if GI upset15 | Manufacturer recommends CBC and chemistry panel before and periodically during treatment in dogs |
| Serotonin selective reuptake inhibitors (e.g., fluoxetine) | Intermittent inappetence39; shortened REM sleep in laboratory cats at doses of 2.5 mg/kg51; increased liver enzymes in humans | Monitor appetite and body weight; consider baseline liver enzyme assessment |
| Tricyclic antidepressants (e.g., amitriptyline, clomipramine) | Lethargy4,22,50 and diminished coat quality4; anticholinergic effects (urine retention)37; cardiac conductivity disturbances in humans (seen in healthy cats only with overdose)13; hepatopathy in humans | Consider baseline CBC, chemistry panel, and cardiac evaluation; consider periodic liver enzyme assessment; monitor urine and fecal output |
| Tramadol | Dysphoria54 | Data limited; safety monitoring is primarily clinical at this time |
ALT, Alanine transaminase; AZT, azidothymidine; CBC, complete blood count; FeLV, feline leukemia virus; FIV, feline immunodeficiency disease; IFN, interferon; PCV, packed cell volume; REM, rapid eye movement.
* Monitoring therapy with glucocorticoids, chlorambucil, and chemotherapeutic agents is discussed above and in Chapter 28, respectively.
Pharmacodynamic Monitoring
Specific physiologic end points (pharmacodynamic measures) are helpful in assessing success of therapy for certain drugs. In cats, these drugs include amlodipine, erythropoietin, methimazole, phosphate binders, and potassium supplements. Efficacy and safety monitoring for these drugs is discussed below, and recommendations are summarized in Table 36-3.
TABLE 36-3 Summary of Monitoring Recommendations for Drugs with Measurable Pharmacodynamic Parameters*
| Drug or Drug Class | Efficacy Monitoring | Safety Monitoring |
|---|---|---|
| Amlodipine | Measure blood pressure 7 days after treatment initiation or dose changes; timing of BP measurement with respect to medication not important | Measure serum K periodically during treatment, especially if concurrent CKD |
| Monitor for clinical signs of hypotension (weakness) if cat is taking multiple agents expected to lower BP | ||
| Erythropoietin (recombinant human); darbepoetin | Measure PCV every 7-14 days until normalized (25%-30%), then monthly if patient is clinically stable | Monitor for development of anemia (due to anti-rHuEPO antibodies) every 14 days for first 60-90 days |
| Monitor blood pressure periodically | ||
| Monitor for vomiting, uveitis, or cutaneous/mucocutaneous lesions | ||
| Methimazole | Measure serum total T4 2-4 weeks after initiating therapy or making dose adjustments, then every 6 months | Measure ALT, ALP, bilirubin, BUN, Cr, and USG periodically throughout treatment (example: every 4-6 months after initial control) |
| Monitor CBC periodically, particularly within first 3 months of treatment, and platelet count before surgery | ||
| Monitor for vomiting or facial excoriation | ||
| Phosphate binders (aluminum salts, calcium salts, chitosan/calcium carbonate, lanthanum carbonate) | Measure serum P concentration monthly until within desired range, then every 2-4 months | Monitor for signs of encephalopathy; monitor CBC periodically for microcytosis (aluminum-containing binders) |
| Measure serum Ca periodically to detect hypercalcemia (calcium-containing products) | ||
| Monitor for constipation (all) | ||
| Potassium supplements | Measure serum K 7-14 days after oral supplementation is initiated or dose changes are made | Discontinue supplementation in oliguric/anuric states (acute-on-chronic renal failure, urinary obstruction) to avoid hyperkalemia |
ALP, Alkaline phosphatase; ALT, alanine transaminase; BP, blood pressure; BUN, blood urea nitrogen; Ca, calcium; CBC, complete blood count; CKD, chronic kidney disease; Cr, creatinine; K, potassium; PCV, packed cell volume; rHuEPO, recombinant human erythropoietin; T4, thyroxine; USG, urine specific gravity.
* Monitoring insulin therapy is discussed in depth in Chapter 24.
Amlodipine
The main objective of efficacy measure for amlodipine, a commonly used antihypertensive in the cat, is blood pressure measurement. Preferred methods for measuring blood pressure in cats, target ranges for blood pressure after treatment, and blood pressure reduction in special situations are discussed in the American College of Veterinary Internal Medicine (ACVIM) Consensus Statement on systemic hypertension2 and in Chapter 20.
In humans, the effects of amlodipine increase gradually in conjunction with plasma levels during 7 to10 days of dosing.30 Unfortunately, no specific information is available regarding the pharmacokinetics of amlodipine in cats. Rechecking blood pressure and making dosage adjustments after 7 days of treatment is probably appropriate based on published studies, although anecdotal reports23 suggest that a clinically significant effect of amlodipine on blood pressure may be seen much sooner (24 to 48 hours). Timing of blood pressure measurement after administration does not appear to be an important factor in evaluating efficacy.53
Side effects reported from three studies of amlodipine in cats8,19,53 included weakness and hypotension (in a patient also receiving propanolol), pruritus, a mild decrease in serum potassium of more than 1 to 2 months following the start of treatment, and need for initiation of potassium supplementation or increase in the dose during the treatment period. Many of the cats in these studies also had some degree of renal dysfunction. Average values for blood urea nitrogen (BUN) and creatinine did not change significantly during the first 1 to 2 months of amlodipine therapy; however, 1 of 10 cats with elevated creatinine in one study became uremic and hypokalemic while on amlodipine.19 Hence, monitoring of serum potassium concentrations and renal values in cats receiving amlodipine is prudent, particularly if chronic renal disease (CRD) has been diagnosed. Owner monitoring for signs of hypotension may be important in cats taking multiple agents expected to lower blood pressure.8
Erythropoietin
Efficacy of therapy with recombinant human erythropoietin (rHuEPO) is documented by measuring the hematocrit (packed cell volume, PCV). This should be done every 1 to 2 weeks during initial therapy. Once PCV has reached 25% to 30% (often by week 4), the frequency of dosing can be reduced.6,24 Thereafter, PCV should be monitored periodically (one author suggests monthly24) in animals receiving erythropoietin, to avoid creating polycythemia and to detect the severe anemia that can result from development of anti-rHuEPO antibodies.
Anemia secondary to antibody development occurred in of five of seven cats treated for more than 180 days in one study,6 4 to 16 weeks after therapy was begun. Darbepoetin, a chemically modified rHuEPO, has not been associated with antibody-positive anemia in human patients to date.5 No data is available on the likelihood of antibody formation with this form of rHuEPO in cats.
Vomiting, uveitis, seizures, and cutaneous hypersensitivity were reported side effects in a group of 11 cats receiving rHuEPO; both cats with seizures were moderately or severely azotemic, and at least one was hypertensive at the time the seizures were noted.6 Hypertension can result from use of erythropoietin, possibly due to increased blood viscosity.24 Erythropoietin treatment increases the demand for iron, and iron deficiency may develop if stores are inadequate. Therefore serum iron should be measured before and during therapy with erythropoietin; supplementation with ferrous gluconate or sulfate may be indicated.
Methimazole
Methimazole therapy is usually monitored by measurement of total thyroxine (T4) concentrations. Normalization of T4 levels in treated hyperthyroid cats typically takes 2 to 4 weeks,55 because the drug inhibits T4 production but does not affect thyroid hormone that has already been synthesized and is either stored in the gland or in circulation. Timing of sampling during the day does not appear to affect results.45 Dosage adjustments may be necessary over time, resulting from growth of the thyroid tumor. Thyroid stimulating hormone (TSH) measurement is also possible, because feline TSH shows cross-reactivity in the canine assay.41 TSH levels are normal to low in cats with adequate thyroid function; elevated TSH levels can indicate overtreatment with antithyroid medication.
Cats with hyperthyroidism and high-normal creatinine levels may progress to mild to moderate azotemia following treatment, regardless of the treatment modality (i.e., medical, surgical, or radioiodine). Development of azotemia typically occurs within the first 30 days following initiation of therapy. Although BUN and creatinine may increase significantly, azotemia is often stable and nonprogressive.25 It is not clear whether careful dose titration to avoid subnormal T4 concentrations affects the development of azotemia, but such titration is possible with methimazole therapy (as opposed to radioiodine or surgery). Indicators of renal function, such as urine specific gravity (USG), urine protein : creatinine (UPC) ratio, glomerular filtration rate (GFR) (measured by exogenous creatinine or iohexol clearance), and the urinary enzyme N-acetyl-beta-D-glucosaminidase have been investigated as monitoring tools. Their ability to predict propensity for renal decompensation has been poor in most studies.20 In particular, USG, although simple and noninvasive to measure, is not necessarily predictive, because some cats with renal insufficiency paradoxically retain the ability to concentrate urine to 1.045 or higher.34
Other reported side effects of methimazole include vomiting and anorexia (11% to 23% of cats), facial and/or cervical excoriation (2% to 15% of cats, depending on study), agranulocytosis, thrombocytopenia (epistaxis, oral hemorrhage), hepatopathy (anorexia, vomiting, icterus), lymphadenopathy, or acquired myasthenia gravis (all in < 3% of cats). The ideal time frame for monitoring blood values to detect bone marrow suppression and hepatopathy is not known, but in one large study36 that documented these effects, bone marrow suppression occurred during the first 3 months of therapy.
Carbimazole, a prodrug for methimazole, is widely used in Europe, and is being considered for approval in the United States. A sustained-release product, Vidalta (Intervet/Schering-Plough Animal Health, Summit, NJ), allowing once-daily dosing at 10 to 15 mg/cat/day, PO, has been approved in Europe.14 Monitoring recommendations for carbimazole are similar to those for methimazole; however, more rapid normalization of serum T4 (mean, 5.7 days after the start of treatment) may be achieved with carbimazole at a dosage of 5 mg/cat, PO, every 8 hours.32
Phosphate Binders
Phosphate binders used in cats include aluminum or calcium salts, chitosan/calcium carbonate, sevelamer hydrochloride, and lanthanum carbonate. For most of these compounds, the measurable efficacy indicator is serum phosphate concentration. To date, there are no controlled studies demonstrating the most appropriate values for serum phosphate in cats with renal disease. Suggestions for target values (derived from human medicine) can be found in a recent roundtable discussion on the management of hyperphosphatemia in dogs and cats.9 After initial achievement of target phosphate levels through dose titration, periodic reassessment is necessary because progression of renal disease or changes in diet may alter serum phosphate concentrations with time. One author recommends measuring serum phosphate concentration monthly after starting therapy until phosphate is in the lower portion of the reference range, then every 2 to 4 months.21
A chitosan/calcium carbonate product labeled for veterinary use as a food additive (Epakitin;Vétoquinol, Fort Worth, Tex.) has been shown to lower plasma parathyroid hormone (PTH) concentrations in cats with chronic renal disease. Titrating dose to normalize PTH levels has been recommended,21 although a dose-response relationship of phosphate binders on PTH has not been established.
There are few studies evaluating the safety of phosphate binders in animals. In humans, all phosphate binders can cause gastrointestinal side effects (e.g., constipation, GI upset). In addition, aluminum-containing phosphate binders are no longer used in human medicine because of occurrences of aluminum toxicity. This complication has also been reported in two dogs with renal disease, after 2 to 6 weeks on doses of 126 to 200 mg/kg/day of aluminum hydroxide. Both dogs had elevated serum aluminum levels; signs of toxicity included lethargy progressing to obtundation and recumbency, with decreased reflexes.48 Progressive microcytosis preceded the development of neurologic signs in these dogs. For this reason, and because microcytic anemia is also associated with aluminum toxicity in humans, one clinician has advocated serial evaluation of red blood cell (RBC) indices in all animals receiving aluminum-containing phosphate binders.12
Hypercalcemia is a concern with calcium-containing phosphate binders and has been observed in cats taking Epakitin.21 Sevelamer or lanthanum salts may be alternatives in cats with hypercalcemia, although sevelamer has not been used extensively in cats. No adverse effects were observed with administration of a liquid formulation of lanthanum carbonate to 10 cats with renal disease during a 6-month period.46
Potassium Supplements
Therapy with oral potassium supplements (potassium gluconate, potassium citrate) is very safe and can be monitored by sequential measurement of serum potassium concentration. One author38 suggests rechecking potassium concentrations every 7 to 14 days during initial therapy. Once potassium concentration has normalized, frequency of rechecks depends on the severity of concurrent disease. The development of hyperkalemia in cats on potassium supplements is reportedly rare as long as urine production (and, therefore, urinary potassium excretion) is adequate.7
Pharmacokinetic Monitoring
Measurement of plasma drug concentration is indicated for those drugs that
1 Exhibit inconsistent absorption, elimination, or interaction characteristics, leading to variation in plasma concentration among individuals
2 Show a correlation between drug concentration and toxicity and/or efficacy
3 Have a commercially available assay, validated for veterinary patients, through which results can be obtained in a timely manner52
Cyclosporine is gaining popularity as a treatment for allergic and immune-mediated diseases. As in other species, cyclosporine absorption is unpredictable in the cat.29 Theophylline has been a recommended treatment for feline bronchitis. For both cyclosporine and theophylline, measurement of plasma drug concentrations may be indicated when lack of efficacy or toxicity is suspected, despite an adequate dose, or when the formulation of the drug is changed.
The main utility of therapeutic drug monitoring in cats at this time is in monitoring therapy with anticonvulsants, particularly phenobarbital. Monitoring of anticonvulsant therapy is discussed in depth in Chapter 27. Recommendations for measurement of aminoglycoside, cyclosporine, phenobarbital, and theophylline levels are found in Table 36-4.
TABLE 36-4 Therapeutic Drug Monitoring in Cats
| Drug | Sample Preparation and Timing35* | Target Plasma Concentrations35 |
|---|---|---|
| Aminoglycosides | 0.5 mL serum or plasma, shipped on ice; sample at 1, 2, and 4 hours after dose for clearance measurements | Amikacin: peak 40 µg/mL; trough <0.8 µg/mL |
| Gentamicin: peak 20 µg/mL; trough <0.27 µg/mL | ||
| Cyclosporine | 1 mL whole blood in EDTA tube, shipped on ice; sample at least 48 hours after beginning or altering therapy; trough or peak (2 hours after dosing) samples may be appropriate | Depends on disease; in general, trough should be 300-600 ng/mL |
| Phenobarbital | 0.5 mL serum or plasma; sample at steady state (7-14 days after first dose), any time during dosing interval | 15-40 µg/mL (extrapolated from dogs); one author recommends 20-30 µg/mL for cats44 |
| Theophylline | 0.5 mL serum, shipped on ice; sample at trough; add peak sample for assessment of clearance (timing of peak varies with formulation) | 5-20 µg/mL expected to be therapeutic (extrapolated from humans) |
EDTA, Ethylenediaminetetraacetic acid.
* Verify specific instructions for sample handling with laboratory.
1 Adams LG. Updates in management of chronic kidney disease, Western Veterinary Conference 2009. [proceedings online]. Available at http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=wvc2009&PID=pr50998&O=VIN, 2009. Accessed February 11
2 Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21:542.
3 Caylor KB, Cassimatis MK. Metronidazole neurotoxicosis in two cats. J Am Anim Hosp Assoc. 2001;37:258.
4 Chew DJ, Buffington CA, Kendall MS, et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc. 1998;213:1282.
5 Cournoyer D, Toffelmir EB, Wells GA. Anti-erythropoietin antibody-mediated pure red cell aplasia after treatment with recombinant erythropoietin products: recommendations for minimization of risk. J Am Soc Nephrol. 2004;15:2728.
6 Cowgill LD, James KM, Levy JK, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc. 1998;212:521.
7 Dow SW, Fettman MJ. Chronic renal disease and potassium depletion in cats. Sem Vet Med Surg. 1992;7:198.
8 Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Sm An Pract. 2001;42:122.
9 Elliot J, Brown SA, Cowgill LD, et al. Phosphatemia management in the treatment of chronic kidney disease, a roundtable discussion. January 10, 2006. Available at http://www.vetoquinol.ca/documents/Quoi%20de%20neuf/Articles/Round%20table%20discussion.pdf, 2010. Accessed January 15
10 European Medicines Agency, Veterinary Medicines. European Public Assessment Report, Virbagen Omega. Available at http://www.ema.europa.eu/vetdocs/PDFs/EPAR/virbagenomega/V-061-en1.pdf, 2010. Accessed January 15
11 Fernandes NF, Geller SA, Fong T. Terbinafine hepatotoxicity: case report and review of the literature. Am J Gastroenterol. 1998;93:459.
12 Fischer J. Dealing with renal hyperphosphatemia: the good, the bound, and the ugly, Veterinary Information Network Rounds transcript. June 22, 2008 [website]. Available at http://www.vin.com/Members/CMS/Rounds/default.aspx?id=887, 2010. Accessed February 1
13 Follmer CH, Lum BK. Protective action of diazepam and of sympathomimetic amines against amitriptyline-induced toxicity. J Pharm Exp Ther. 1982;222:424.
14 Frenais R, Rosenberg D, Burgaud S, et al. Clinical efficacy and safety of a once-daily formulation of carbimazole in cats with hyperthyroidism. J Small Anim Pract. 2009;50:510.
15 Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy, and palatability of oral meloxicam at 0.01-0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg. 2008;10:235.
16 Hartmann K, Donath A, Kraft W. AZT in the treatment of feline immunodeficiency virus infection, part 2. Feline Pract. 1995;23:13.
17 Haschek WM, Weigel RM, Scherba G, et al. Zidovudine toxicity to cats infected with feline leukemia virus. Fund Appl Toxicol. 1990;14:764.
18 Helton KA, Nesbitt GH, Caciolo PL. Griseofulvin toxicity in cats: literature review and report of seven cases. J Am Anim Hosp Assoc. 1986;22:453.
19 Henik RA, Snyder PS, Volk LM. Treatment of systemic hypertension in cats with amlodipine besylate. J Am Anim Hosp Assoc. 1997;33:226.
20 Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med. 2009;23:806.
21 Kidder A, Chew D. Treatment options for hyperphosphatemia in feline CKD: what’s out there? J Feline Med Surg. 2009;11:913.
22 King JN, Steffan J, Heath S, et al. Determination of the dosage of clomipramine for the treatment of urine spraying in cats. J Am Vet Med Assoc. 2004;225:881.
23 Kittleson MD, Kienle RD, editors. Small animal cardiovascular medicine, ed 1. St Louis: Mosby. 1998:445.
24 Langston CE, Reine NJ, Kittrell D. The use of erythropoietin. Vet Clin North Am Small Anim Pract. 2003;33:1245.
25 Langston CE, Reine NJ. Hyperthyroidism and the kidney. Clin Tech Sm Anim Pract. 2006;21:17.
26 Levy JK. Ataxia in a kitten treated with griseofulvin. J Am Vet Med Assoc. 1991;198:105.
27 Mancianti F, Pedonese F, Zullino C. Efficacy of oral administration of itraconazole to cats with dermatophytosis caused by Microsporum canis. J Am Vet Med Assoc. 1998;213:993.
28 McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35:1672.
29 Mehl ML, Kyles AE, Craigmill AL, et al. Disposition of cyclosporine after intravenous and multi-dose oral administration in cats. J Vet Pharmacol Therap. 2003;26:349.
30 Michel T. Treatment of myocardial ischemia. In: Brunton LL, editor. Goodman and Gilman’s the pharmacological basis of therapeutics. ed 11. Chicago: McGraw-Hill; 2006:823.
31 Miller WH, Scott DW. Efficacy of chlorpheniramine maleate for management of pruritus in cats. J Am Vet Med Assoc. 1990;197:67.
32 Mooney CT, Thoday KL, Doxey DL. Carbimazole therapy of feline hyperthyroidism. J Sm Anim Pract. 1992;33:228.
33 Olson EJ, Morales SC, McVey AS, et al. Putative metronidazole toxicosis in a cat. Vet Pathol. 2005;42:665.
34 Osborne CA, Stevens JB, Lulich JP, et al. A clinician’s analysis of urinalysis. In: Osborne CA, Finco DR, editors. Canine and feline nephrology and urology. ed 1. Baltimore: Williams & Wilkins; 1995:141.
35 Papich MG. Therapeutic drug monitoring. In: Papich MG, Riviere JE, editors. Veterinary pharmacology and therapeutics. ed 9. Ames, Iowa: Wiley-Blackwell; 2009:1323.
36 Peterson ME, Kintzer P, Hurvitz AI. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med. 1988;2:150.
37 Pfeiffer E, Guy N, Cribb A. Clomipramine-induced urinary retention in a cat. Can Vet J. 1999;40:265.
38 Plotnick A. Feline chronic renal failure: long-term medical management. Compend Contin Educ Pract Vet. 2007;29:342.
39 Pryor PA, Hart BL, Cliff KD, et al. Effects of a selective serotonin reuptake inhibitor on urine spraying behavior in cats. J Am Vet Med Assoc. 2001;219:1557.
40 Quimby JM, Gustafson DL, Samber BJ, et al. The pharmacokinetics of mirtazapine in healthy cats [abstract]. J Vet Intern Med. 2009;23:767.
41 Rayalam S, Eizenstat LD, Davis RR, et al. Expression and purification of feline thyrotropin (fTSH): immunological detection and bioactivity of heterodimeric and yoked glycoproteins. Dom Anim Endocrin. 2006;30:185.
42 Rottman JB, English RV, Breitschwerdt EB, et al. Bone marrow hypoplasia in a cat treated with griseofulvin. J Am Vet Med Assoc. 1991;198:429.
43 Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med. 2005;19:155.
44 Rusbridge C. Diagnosis and control of epilepsy in the cat. In Practice. 2005;27:208.
45 Rutland BE, Nachreiner RF, Kruger JM. Optimal testing for thyroid hormone concentration after treatment with methimazole in healthy and hyperthyroid cats. J Vet Intern Med. 2009;23:1025.
46 Schmidt BH, Murphy M. A study on the long-term efficacy of RenalzinTM (Lantharenol® suspension 20%) in cats with experimentally induced chronic kidney disease [poster]. Proc 19th Eur Coll Vet Intern Med Congress. 2009.
47 Scott DW, Rothstein E, Beningo KE, et al. Observations on the use of cyproheptadine hydrochloride as an antipruritic agent in allergic cats. Can Vet J. 1998;39:634.
48 Segev G, Bandt C, Francey T, et al. Aluminum toxicity following administration of aluminum-based phosphate binders in 2 dogs with renal failure. J Vet Intern Med. 2008;22:1432.
49 Sekis I, Ramstead K, Rishniw M, et al. Single-dose pharmacokinetics and genotoxicity of metronidazole in cats. J Feline Med Surg. 2009;11:60.
50 Seksel K, Lindeman MJ. Use of clomipramine in the treatment of anxiety-related and obsessive-compulsive disorders in cats. Aust Vet J. 1998;76:317.
51 Slater IH, Jones GT, Moore RA. Inhibition of REM sleep by fluoxetine, a specific inhibitor of serotonin uptake. Neuropharmacology. 1978;17:383.
52 Smith JA. What is the role of therapeutic drug monitoring in antifungal therapy? Curr Infect Dis Rep. 2009;11:439.
53 Snyder PS. Amlodipine: a randomized, blinded clinical trial in 9 cats with systemic hypertension. J Vet Intern Med. 1998;12:157.
54 Steagall PV, Taylor PM, Brondani JT, et al. Antinociceptive effects of tramadol and acepromazine in cats. J Feline Med Surg. 2008;10:24.
55 Trepanier LA. Pharmacologic management of feline hyperthyroidism. Vet Clin North Am Small Anim Pract. 2007;37:775.
56 Veterinary Information Network discussion. mirtazapine toxicity? September 14, 2009 [website]. Available at http://www.vin.com/Members/boards/discussionviewer.aspx?DocumentId=4113030, 2010. Accessed January 15
57 Vlaminck KM, Engelen MA. An overview of pharmacokinetic and pharmacodynamic studies in the development of itraconazole for feline Microsporum canis dermatophytosis. In: Hillier A, Foster AP, Kwochka K, editors. Advances in veterinary dermatology, vol 5. Ames, Iowa: Blackwell Publishing; 2005:130.
58 Whitney BL, Broussard J, Stefanacci JD. Four cats with fungal rhinitis. J Feline Med Surg. 2005;7:53.
59 Zeidner NS, Myles MH, Mathiason-DuBard CK. Alpha interferon (2b) in combination with zidovudine for the treatment of presymptomatic feline leukemia virus-induced immunodeficiency syndrome. Antimicrob Agents Chemother. 1990;34:1749.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree