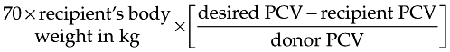CHAPTER 25 Hematology and Immune-Related Disorders
Blood and immune diseases are relatively common in cats and often caused by infectious agents. Many of the presenting signs are nonspecific, requiring a detailed and logical investigation into their cause. Understanding normal physiology is important in recognizing disease. This chapter covers some diagnostic techniques useful in evaluating cats with blood disease. Important, non-neoplastic blood and systemic immune dysfunction is discussed, with an emphasis on diagnosis and treatment. Specific immune disorders of the skin and joints are covered in Chapters 22 and 26, and neoplastic diseases are discussed in Chapter 28.
Diagnostic Techniques
Bone Marrow Collection
Indications
Indications for obtaining a sample of bone marrow include the presence of an unexplained nonregenerative anemia, neutropenia, thrombocytopenia, or combination of cytopenias (Box 25-1). Bone marrow collection can be used to stage neoplasia or determine the etiology of hypercalcemia or hypergammaglobulinemia that may be caused by lymphosarcoma or multiple myeloma. Although most healthy cats do not have visible iron stores in the bone marrow, the presence of iron here will eliminate iron deficiency as a cause of anemia.34 Additional indications for collecting bone marrow include the inappropriate presence of immature hematopoietic cells in the circulation, unexplained leukocytosis or thrombocytosis, and dysplastic changes in the circulating blood cells. Routine evaluation of the bone marrow is not helpful in sorting out the causes of absolute erythrocytosis (polycythemia) because the erythroid morphology of the marrow appears the same in all cases (erythroid hyperplasia).47
BOX 25-1 Indications for Sampling Bone Marrow
Abnormal Peripheral Blood Findings
Equipment and Supplies
Several types of bone marrow needles are available (Figure 25-1). An 18-gauge needle is an appropriate size for collecting marrow from a cat. The Jamshidi and Rosenthal needles are made of stainless steel and can be heat sterilized. The Illinois needle contains plastic and requires gas or cold sterilization. The presence of a stylet in the needle keeps the lumen from becoming plugged with a core of cortical bone at the beginning of the procedure. The stylet must be completely in place until the actual sample is collected, or a frustrating obstruction of the needle will occur. For hospitals without a bone marrow needle, an 18-gauge venipuncture needle may be substituted. Because there is no stylet to keep the lumen open, an obstruction of the venipuncture needle is likely. This means a second needle will be needed, and dexterity will be required to find the hole in the cortical bone made by the first needle.
Bone marrow clots readily when collected. The use of an anticoagulant is recommended so that there is no rush to process the sample after collection. A 2.5% solution of ethylenediaminetetraacetic acid (EDTA) can be made by injecting 0.35 mL of sterile saline into a 3-mL lavender-top EDTA blood collection tube. The contents are withdrawn and injected into a second EDTA tube.71 The resulting 0.5 mL volume is placed in a 12-mL syringe and should be adequate for preventing coagulation of the collected marrow sample.
Collection Sites
Bone marrow can be collected from one of three sites: the proximal humerus (Figure 25-2), the iliac crest, or the femur (Figure 25-3). The proximal humerus is easily accessible, has little overlying tissue, and offers a large surface for needle placement. The greater tubercle is palpated, and the needle inserted into the flat surface of the craniolateral humerus just distal to the tubercle. The needle is inserted in a craniomedial direction perpendicular to the long axis of the bone.
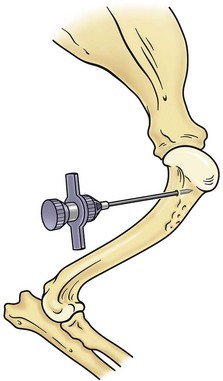
FIGURE 25-2 Bone marrow collection site from the proximal humerus.
(Redrawn from Grindem CB: Bone marrow biopsy and evaluation, Vet Clin North Am 19:674, 1989.)
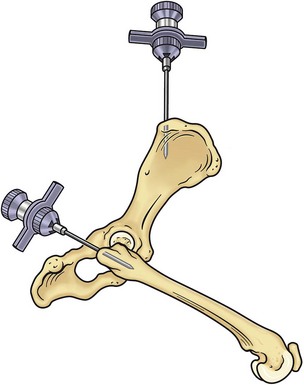
FIGURE 25-3 Bone marrow collection sites from the iliac crest and the proximal femur.
(Redrawn from Grindem CB: Bone marrow biopsy and evaluation, Vet Clin North Am 19:673, 1989.)
Core Biopsy
If an aspiration of the bone marrow has been performed, the needle should still be in place. If not, the veterinarian should follow the instructions for performing an aspiration of bone marrow without aspirating any bone marrow. Collecting a core sample of bone marrow from a separate site may increase the likelihood of identifying metastatic neoplasia.71
Cross-Match
A quick means of performing a major cross-match is to mix 2 drops of plasma from the recipient with 1 drop of anticoagulated blood from the donor on a slide at room temperature.35 The opposite will be a minor cross-match. Development of macroscopic agglutination within a minute suggests the presence of anti-A alloantibodies in the plasma sample of the recipient (major cross-match) or donor (minor cross-match). In either case the blood is incompatible. Autoagglutination can make interpretation of the test difficult. Running a control test using saline instead of plasma may help with interpretation.
For hospitals performing frequent transfusions, a standardized gel agglutination test is available for in-hospital use. Although more time consuming than the previously described method, the gel test is less vulnerable to operator interpretation error. Because it is stable, the test result can be saved and reviewed at a later time if needed.118 Two commercially available products include the DiaMed-ID cross-match gel (DiaMed, Switzerland) and the Rapid Vet-H companion animal cross-match gel (DMS Laboratories, Flemington, New Jersey). More rigorous and time-consuming methods involving washing, centrifuging, and incubating samples have been published.25,42
Slide Agglutination Test
A positive, properly performed slide agglutination test suggests the presence of antibody-coated erythrocytes and negates the need for performing a direct Coombs’ test. It is important to differentiate erythrocyte clumping caused by autoagglutination from that caused by rouleaux formation. These types of erythrocyte clumping are differentiated by washing the cells with saline, which will reliably break up clumps formed by rouleaux. A quick method of performing the test is to mix a drop of EDTA anticoagulated blood on a slide with 2 to 5 drops of 0.9% NaCl followed by a gross and microscopic examination of the sample.54 The “stacked coins” appearance characteristic of rouleaux formation (Figure 25-4) will disperse, whereas the random or rosette clumping of autoagglutination will not (Figure 25-5). If the test is negative, a direct Coombs’ test should be requested. An important limitation to this test is its inability to separate primary from secondary immune-mediated disease.
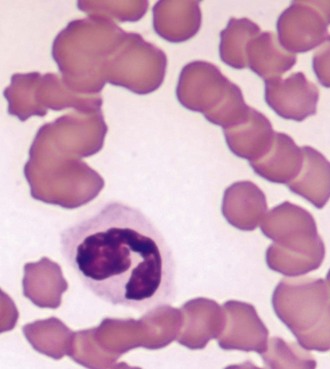
FIGURE 25-4 The “stack of coins” arrangement associated with rouleaux formation.
(Courtesy Rick Cowell.)
Erythrocyte Physiology And Diagnostic Evaluation
The production of erythrocytes by the bone marrow is influenced by the hormone erythropoietin (EPO), which is produced by fibroblasts adjacent to the renal tubules near the corticomedullary junction in response to decreased local oxygen tension.129 Increased EPO production begins within minutes of onset of hypoxia, with maximal production occurring 24 hours later. Colony-forming-unit erythrocytes in the bone marrow respond to increased concentrations of EPO by increasing production, maturation, and release into circulation of new red cells. Increasing numbers of new circulating erythrocytes will not be apparent for at least 2 or 3 days.
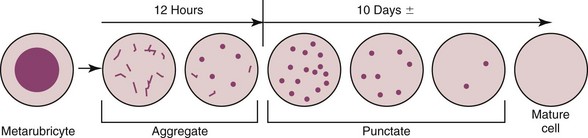
It is important to realize that these two types of reticulocytes are not different cells but rather a progression in the maturation of the erythrocyte (Figure 25-6). As the anemia becomes more severe, younger reticulocytes are released in an attempt to increase the number of oxygen-carrying red cells. The result is an increase in the number of aggregate reticulocytes in the circulation. Because they mature so quickly to punctate reticulocytes, the presence of increased numbers of circulating aggregate reticulocytes suggests ongoing hypoxia. An important concept regarding feline anemia is that an increase in the numbers of aggregate reticulocytes (above the reference range for the laboratory) is required before a moderate to severe anemia is considered regenerative. Unless the anemia is mild, and the more immature aggregate reticulocytes are not required, the presence of punctate reticulocytes alone is not evidence of regeneration. In cats the absolute number of aggregate reticulocytes is a more reliable indicator of regeneration than the corrected reticulocyte percentage or the reticulocyte production index.54
EPO also stimulates hemoglobin synthesis. Feline hemoglobin is unique in that it has less affinity for oxygen than the hemoglobin found in other species; consequently, oxygen is more easily released to the tissues. This may be one explanation for why the packed cell volume (PCV) and hemoglobin concentration in the normal cat are lower than those of normal dogs.54 In a healthy cat the production and removal of erythrocytes is balanced. The life span of the normal, mature feline erythrocyte is approximately 73 days, after which they are removed from circulation by macrophages in the spleen, and the heme and iron recycled.
Quantitative Erythrocyte Parameters
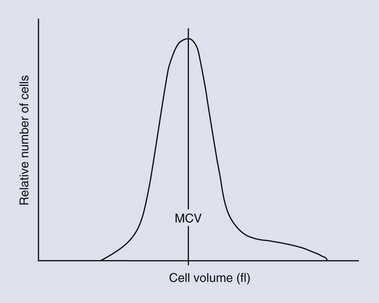
Erythrocytes can be classified by their size and hemoglobin concentration on the basis of quantitative parameters such as the mean corpuscular volume (MCV, the average cell size), the red cell distribution width (RDW), and the mean corpuscular hemoglobin concentration (MCHC). Macrocytosis, normocytosis, and microcytosis refer to cell size above, within, or below the reference range, respectively. The RDW is derived from the cell numbers versus cell volume histogram (Figure 25-7); an increase in RDW indicates a greater than normal variation in cell size. The RDW may be artifactually affected by the overlap in size between feline platelets and red cells. Normochromia and hypochromia refer to MCHC within or below the reference range, respectively. Hemoglobin makes up approximately 33% of the volume of the cell. Erythrocytes cannot carry more hemoglobin in their cytoplasm than normal, so they cannot be hyperchromic. An increased MCHC is usually associated with hemolysis, either a result of disease or of improper venipuncture or sample handling. A change in any of these parameters requires a review of a blood smear for an explanation.126
Qualitative Erythrocyte Parameters
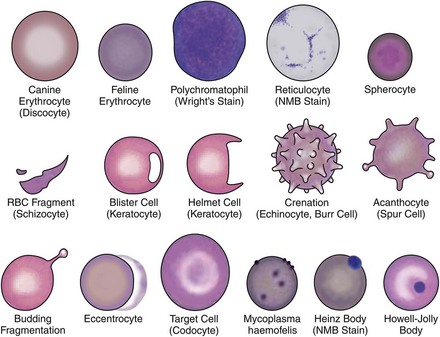
Qualitative erythrocyte parameters are based on a blood smear evaluation. Increased variations in cell size, color, and shape are known as anisocytosis, polychromasia, and poikilocytosis, respectively. Anisocytosis is present if there is a combination of normal-size cells along with an appreciable number of larger or smaller cells. Anisocytosis may result in an increased RDW. The larger cells are often reticulocytes, although infection with the feline leukemia virus (FeLV) can result in larger cells without increased reticulocyte numbers. Polychromasia is usually due to the presence of increased numbers of aggregate reticulocytes and indicates regeneration.126 Lack of polychromasia, however, does not rule out regeneration.18 Variations in cell shape may be artifactual or due to disease (Figure 25-8). Echinocytes are crenated red cells with uniform, often pointy, projections. They are usually artifacts but are important to recognize; when the projections are viewed end on, they may appear as small rings and mimic the ring form of hemoplasmosis (e.g., Mycoplasma haemofelis). Acanthocytes are similar to echinocytes but have fewer and more rounded projections. They are frequently present in cats with hepatic disease. Erythrocyte fragments, such as schistocytes or keratocytes, are the result of cell trauma. When there are many fragments, the presence of turbulent blood flow or microangiopathic disorders such as hemangiosarcoma or disseminated intravascular coagulation (DIC) should be considered. Iron deficiency may also cause increased fragmentation.18 Spherocytes are smaller cells that are the product of immune-mediated removal of antibody-coated parts of the erythrocyte membrane, after which the cell is reconfigured into a sphere. Because normal feline erythrocytes are small and lack central pallor, spherocytosis in this species is difficult or impossible to appreciate and identification is best left to an experienced veterinary hemocytologist.
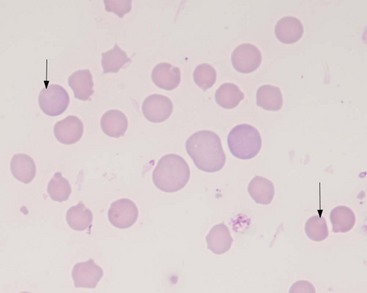
Microagglutination and rouleaux formation may be visible on blood smears. Agglutination appears as a random, disorganized clumping of cells not dispersed by the addition of saline. True autoagglutination indicates an immune-mediated disease affecting the erythrocyte. Rouleaux formation looks similar to a stack of coins (see Figure 25-4) and will disperse after the addition of saline (see Figure 25-5). Circulating monocytes may phagocytose antibody-covered red cells; this is called erythrophagocytosis. Although not observed very often, erythrophagocytosis also suggests the presence of immune-mediated red cell damage. Heinz bodies are areas of oxidatively denatured hemoglobin within the cell (discussed later). The altered hemoglobin is pushed off to one side and often seen as a projection off the surface of the cell membrane. Heinz bodies stain dark with new methylene blue stain, somewhat clear with Diff-Quik, and the same as the cytoplasm with Wright’s stain18 (Figures 25-9 and 25-10). Howell–Jolly bodies are intracytoplasmic remnants of nuclear material found in erythrocytes that may mimic red cell parasites. Blood smear examination is an essential part of a CBC, particularly when it comes to evaluating the erythron. There is no other way to identify the morphologic changes in red cells that can yield clues to the etiology of erythrocyte disease. Without a blood smear evaluation, a CBC is incomplete.
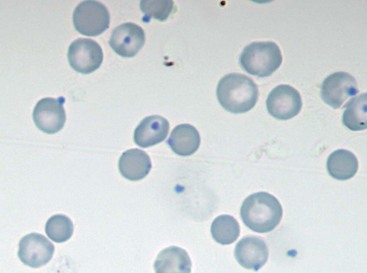
FIGURE 25-9 Heinz bodies appear as dark-stained structures in this new methylene blue–stained smear of feline blood.
(Courtesy Rick Cowell.)
Blood Types
There are three well-known, clinically important blood groups in cats: A, B, and AB. Another potentially important group called Mik has recently been identified.124 The blood groups are genetically determined erythrocyte surface antigens. The A-allele is dominant over the b-allele so that cats with genotypes A/A and A/b will be type A, whereas only the homozygous b/b will have the type B phenotype. A third allele, Ab, occurs rarely and is said to be recessive to the A-allele and dominant to the b-allele, although controversy exists regarding the exact inheritance. A and B antigens are produced on the same red cell only in cats with the genotypes Ab/b or Ab/Ab.35 A more in-depth discussion of feline blood types has recently been published.7 Blood typing can be performed by a diagnostic laboratory using various methods or in the hospital with a card typing system (DMS RapidVet-H [feline], DMS Laboratories). If the card-typing system is used, type AB and type B results should be confirmed by a referral laboratory because some cross-reactions have been known to occur.104 A recently introduced option for patient-side blood typing is the gel column agglutination test (DiaMed-Vet feline typing gel, DiaMed, Switzerland). This test is easier to interpret than the card method, although it requires a specially designed centrifuge that may be cost prohibitive in some settings.118 An evaluation of various blood typing methods for the cat concluded that the gel column test is reliable compared with the gold standard, the Penn tube assay.104
Knowledge of this system is important in the prevention of transfusion reactions and neonatal isoerythrolysis. Cats with type B blood have anti-A antibodies with strong hemolytic potential. Even a small volume of type A or AB blood administered to a type B cat can cause potentially life-threatening hemolysis within minutes of the transfusion.36 Hemolysis of type B blood administered to a type A cat will result in a reduced life span of the transfused erythrocytes, but severe reactions are uncommon.42 Ingestion of colostrum from a type B queen by a type A newborn results in absorption of anti-A alloantibodies and subsequent rapid hemolysis of the kitten’s erythrocytes. This is known as neonatal isoerythrolysis and occurs only when type A or AB kittens are born to a type B queen.
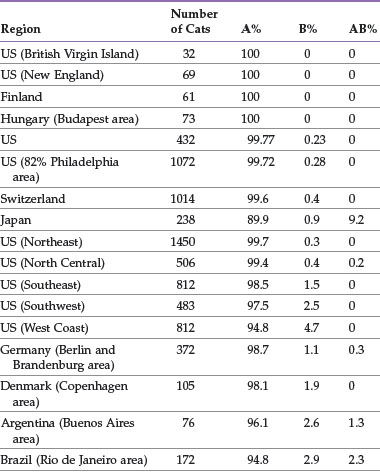
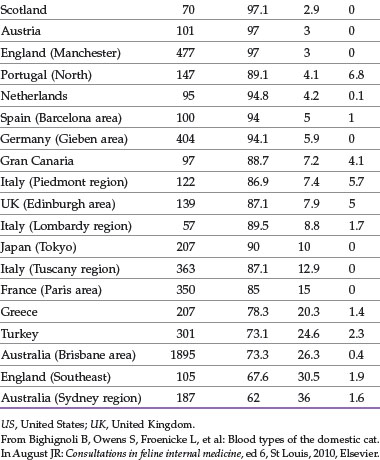
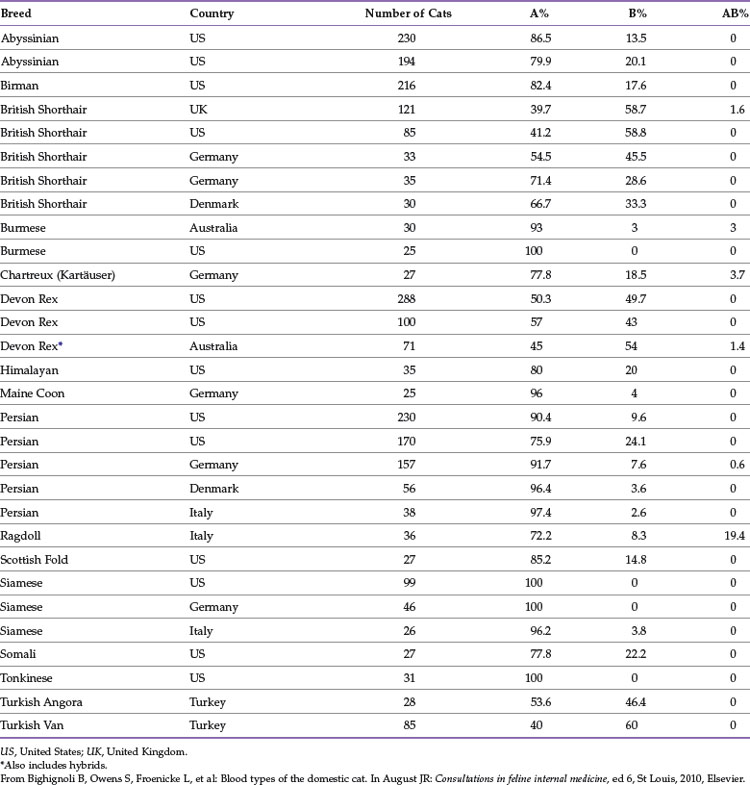
The distribution of blood types varies by geographic region and breed (Tables 25-1 and 25-2). Type A is the most common type among cats. There is, however, a geographic variation in the number of type B domestic shorthair cats. More than 10% of the domestic shorthair cats in Australia, France, Greece, India, Italy, Japan, Turkey, and some regions of England are type B. Distribution of blood types among pedigreed breeds does not vary as much by location because of the international exchange of breeding cats. More than 30% of British Shorthair cats, Cornish and Devon Rex cats, and Turkish Angora or Vans have type B blood. In contrast, Siamese and related breeds are almost exclusively type A. Ragdoll cats appear to be unique with regard to blood types. Approximately 3% of Ragdoll cats are discordant when genotyping is compared to serology, necessitating further investigation in this breed.7 The AB blood type is very rare, and the frequency of the Mik blood type is unknown. The presence of erythrocyte antigens in addition to the A and B groups may explain why transfusion compatibility is not guaranteed by blood typing; cross-matching is recommended before any transfusion.124 Breeding queens, along with blood donors and, if possible, blood recipients, should be blood typed.
Clinical Evaluation of Cats with Anemia
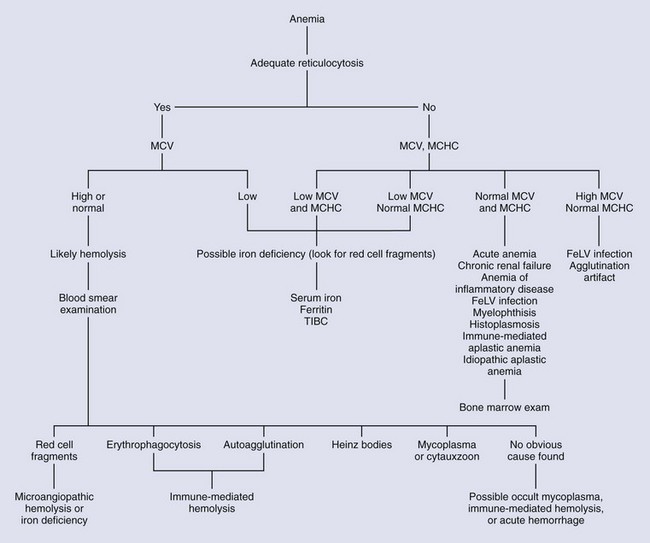
When presented with a pale cat, the first diagnostic step is to measure the PCV and total plasma protein concentration. If the PCV is normal, the veterinarian should look for other causes of pallor. If the PCV is low, the next step is to determine if the anemia is regenerative or nonregenerative (Figure 25-11). The single, best indicator of regeneration is an increase in the absolute aggregate reticulocyte count.126 The severity of anemia should not be assessed until any volume deficits have been corrected. A CBC with a platelet and aggregate reticulocyte count and a blood smear examination will provide evidence of regeneration as long as sufficient time has elapsed since the initial insult. If the protein content is low, acute bleeding should be suspected. Additional tests to consider include a slide agglutination test, a direct Coombs’ test, testing for retroviral infection, and a polymerase chain reaction (PCR) test for hemotrophic Mycoplasma. Other tests to consider include thoracic and abdominal radiography and abdominal ultrasonography, a serum biochemical profile, urinalysis, and coagulation profile. If the anemia is nonregenerative, evaluation of the bone marrow may be required to make an etiologic diagnosis. An attempt should be made to biopsy any masses identified during the evaluation. Examination and sampling of the gastrointestinal mucosa may be required to diagnose causes of blood loss from this system. To differentiate anemia of inflammatory disease from iron deficiency anemia, serum iron, ferritin, and transferrin (total iron-binding capacity) will have to be measured. By following a logical, ordered diagnostic approach to anemia, the veterinarian can often make an etiologic diagnosis, allowing specific therapy to be instituted.
Supportive Care For Cats With Anemia
Specific treatment for an anemic cat can be attempted only after the cause has been identified. Until that time, supportive care is essential. Bleeding should be controlled to prevent further blood loss. Home care while awaiting test results may be adequate if the anemia is mild. Avoiding stressful situations, such as excessive handling, barking dogs, or fractious cats, will help reduce oxygen requirements. Correction of volume contraction may improve the patient’s attitude and appetite. Intravenous fluids may be necessary if volume depletion is significant. Concerns regarding reduction of oxygen-carrying capacity by reducing the PCV with fluid therapy are probably unfounded. The total body hemoglobin and oxygen-carrying ability remains unchanged. However, cats with low plasma protein levels are at risk of edema formation as a result of dilution by aggressive fluid administration. Cats with severe signs related to the anemia, such as respiratory distress or extreme weakness, may require a transfusion. Oxygen administration adds little to the ability to improve tissue hypoxia in anemic patients.35 The low solubility of oxygen in plasma results in a very small increase in the dissolved oxygen content when 100% oxygen is inhaled. In addition, the stress a cat may experience during oxygen administration may be deleterious.
Basic Feline Transfusion Medicine
The indications for the use of blood products are many and include hemorrhage, anemia, hemostatic defects, and hypoproteinemia.42 Many blood products are available or may be prepared, although most veterinary hospitals have hospital cats to use as needed for whole blood donation.
Many blood products are available and have specific uses. Fresh, whole blood contains erythrocytes, platelets, clotting factors, and serum proteins. Storage of whole blood results in the loss of platelets in 2 to 4 hours and clotting factors V and VIII within 24 hours of collection.42 Packed red cells maintain the oxygen-carrying capacity of whole blood in a smaller volume. This product may be used when volume expansion is unwanted, such as anemic cats with heart disease. Fresh frozen plasma contains albumin and all the clotting factors and is used in cats hemorrhaging from coagulation disorders such as liver failure, DIC, or anticoagulant rodenticide toxicity. The use of plasma products to treat hypoalbuminemia is beneficial only in the short-term because transfused albumin rapidly equilibrates with the extravascular space.25 The addition of synthetic colloids may prolong the oncotic effects of a plasma transfusion in these cats.42 Platelet-rich plasma is indicated for cats bleeding from platelet deficiency or dysfunction. Sources for products other than fresh whole blood include a local emergency or referral hospital or a regional veterinary blood bank. Oxyglobin (Biopure, Cambridge, Mass.) is a bovine hemoglobin product containing 130 g/L of hemoglobin that has been licensed for use in dogs. Because no cell membranes are present, there is no antigenicity and the product can be used when compatible blood is unavailable. However, availability of the product has been erratic, and at the time of this writing, it is not available.
Donor cats should be healthy larger cats with a PCV above 35% and fully vaccinated.42 Donors should be blood typed before blood is collected. No abnormal morphologic cell types should be present, and platelet numbers should be in the reference range. The American College of Veterinary Internal Medicine (ACVIM) consensus statement on screening blood donors recommends testing donor cats for M. haemofelis; Candidatus Mycoplasma haemominutum; FeLV antigen; feline immunodeficiency virus (FIV) antibody; and, possibly, Bartonella infections.122 Cats that test positive for FIV antibody should be excluded even if vaccinated against the disease because development of reliable tests to discriminate between antibodies present from a natural infection versus vaccination has been difficult. Heartworm disease cannot be transmitted by blood donation, insofar as the larvae require passage through the mosquito to become infective. Screening for cytauxzoonosis is unnecessary because most cats with the disease are ill. Toxoplasmosis and feline infectious peritonitis have not been documented to be transmitted by transfusion.122 Donor cats should be kept indoors to reduce the risk of exposure to infectious diseases. Healthy cats can donate 10% to 20% of their total blood volume without adverse effects. A cat’s total blood volume is approximately 66 mL/kg. For example, approximately 50 mL of blood (10 mL/kg) can be collected from a 5-kg cat no more often than every 4 to 6 weeks. Subcutaneous fluids should be administered at 2 to 3 times the volume of donated blood. Collection of more than 70 mL of blood from a 5-kg cat can lead to hypovolemia, and the volume should be replaced with intravenous fluids. Many donors resent sitting still long enough to have this volume of blood removed and may require sedation or general anesthesia.
For the treatment of anemia, there are no established levels of PCV below which a cat requires a blood transfusion. The decision to transfuse is instead based on the condition of the patient and assessment of the potential benefits weighed against the risks. Indications that an anemic cat may require a transfusion include respiratory distress, weak pulses, or severe weakness.126 Both the donor and recipient should be blood typed. Even if the blood types are known, cross-matching should be performed before blood administration to prevent incompatible transfusions caused by untested or unknown erythrocyte antigens such as Mik. The half-life of appropriately matched red blood cells in the cat is 29 to 39 days, but for mismatched transfusions the half-life may be a matter of hours. When type B blood is transfused to a type A cat, the life span of transfused red blood cells is only 2 days. When type A blood is transfused to a type B cat, in addition to a potentially severe and fatal reaction, the life span of the transfused cells is only a few hours. A cross-match should be performed again if more than 4 days have elapsed since the last transfusion from the same donor or if another donor is used.
Blood collected for immediate use can be anticoagulated with heparin. Heparin has no preservative properties, and heparinized blood should be used within 8 hours.42 If blood is to be stored for a longer period, citrated anticoagulants should be used. The required blood volume can be collected into a large syringe. If stored blood is used, it should be warmed to room temperature. Blood is administered through a filter connected to a dedicated intravenous line or a line without the presence of calcium-containing fluids. The transfusion is usually administered using gravity flow, although an infusion pump can be used if the manufacturer has stated that it can be used for this purpose. Bacterial contamination is a potential risk, and aseptic techniques for blood collection should be followed. Blood products can be administered intravenously or intraosseously in small patients.
For severely anemic cats the goal of transfusing whole blood is to ameliorate life-threatening decreases in PCV. This can be accomplished by attempting to raise the PCV to over 20%.42 The volume of whole blood required to increase the PCV to a desired level can be calculated by using the following formula:
For cats with incompatible cross-matches or in cases when blood products are unavailable, Oxyglobin (Biopure, Cambridge, Mass.) may be administered at a dose of 5 to 15 mL/kg at a rate of 5 mL/kg/h. Because the product contains no red cells, the hemoglobin concentration is measured to assess the effectiveness of the treatment. Oxyglobin has high colloidal properties, and cats are prone to volume overload with its use. Adverse reactions in cats included vomiting, pulmonary edema, and pleural effusion. In one study of cats receiving Oxyglobin, 20% of cats developing respiratory signs required furosemide or supplemental oxygen during or after treatment. Most of these cats had preexisting heart disease.32 The lower dose should be used cautiously in cats with cardiac disease. The oxygen-carrying effects of Oxyglobin last up to 3 days in circulation.42
Adverse effects of blood transfusions can be immunologic reactions to incompatible blood or nonimmune events and may occur within 1 or 2 hours after the transfusion begins. Occasionally, they may be seen up to 48 hours later.42 In a study of 126 cats that received blood transfusions, 11 cats (8.7%) suffered acute reactions.53 Multiple red blood cell transfusions (either whole blood or packed red blood cells) are also well tolerated in cats and may be critical for survival of some severely ill patients.98 Immune-mediated reactions can include hemolysis, allergic reactions, fever, or graft-versus-host reactions. Bacterial contamination of the blood product, hemolysis, hypocalcemia (from citrate toxicity), hypothermia, hyperammonemia, and volume overload are examples of nonimmune adverse reactions. In either case, the life span of the transfused erythrocytes may be shortened. Some reactions are severe enough to cause death. Despite the best efforts to prevent them, transfusion reactions may still occur. Depending on the severity, therapy may include glucocorticoids, epinephrine, crystalloid intravenous fluids, and discontinuation of the transfusion. Fever is usually mild, requiring no treatment. Furosemide should be administered if volume overload occurs. To prevent hypothermia, the blood product can be warmed to no more than 37° C. If the reaction is relatively mild, the transfusion can be restarted at a slower rate. Cross-matching blood is the best means of preventing immune-mediated transfusion reactions even if the blood type is known for both cats. It is also imperative that blood be collected and administered as aseptically as possible and that cats receiving blood products be carefully monitored.
Erythrocyte Disorders
Regenerative (Responsive) Anemia
History and Physical Examination
The signs associated with anemia are often nonspecific and have been covered in a previous section of this chapter (Clinical Evaluation of Cats with Anemia).
Acute Blood Loss
Early in the course of blood loss, before reticulocytes can be produced and released, the anemia may appear nonregenerative. The physiologic response to volume loss is to shunt blood away from the skin and spleen to protect the heart, brain, and viscera.34 Pallor seen in this situation is not due to anemia but to decreased blood flow to the mucosa. During and immediately after significant blood loss, the PCV may remain normal, insofar as there is loss of both erythrocytes and plasma. A shift of fluid from the interstitial to intravascular space occurs within 12 to 24 hours, diluting the erythrocytes.129 The result is a decrease in the PCV and total protein concentration. These decreases occur earlier if intravenous fluids have been administered. Erythrocyte morphology at this point will be normal, as will the MCV and MCHC. For 3 to 5 days, the anemia will appear nonregenerative and the diagnosis of a blood loss anemia is made on the basis of suspicion, history, physical findings, and a decreased total protein concentration. Once sufficient time has elapsed, reticulocytes appear and the anemia becomes regenerative. If the bleeding is controlled, the transient increase in aggregate reticulocyte numbers is followed by a rise in the number of punctate reticulocytes as the aggregate ones mature.34 If clinical signs are sufficiently severe, a whole blood transfusion should be considered.
If the cause of the bleeding is not determined or controlled, loss of iron stores will lead to an iron deficiency anemia. Gastrointestinal bleeding should be considered if a cause for the blood loss is not obvious. Bleeding gastrointestinal tumors, inflammatory bowel disease, gastric ulcers from overzealous use of nonsteroidal antiinflammatory drugs (NSAIDs), and gastrointestinal parasitism are all potential causes of external blood loss.34 Urinary blood loss is unlikely to cause depletion of iron stores.129 Young kittens infested with fleas can experience significant blood loss, insofar as 100 fleas can consume approximately 1 mL of blood daily.34 This represents about 10% of a 1-kg kitten’s blood volume over a 1-week period.
Immune-Mediated Hemolysis
Dysregulation of the immune system results in a loss of self-tolerance. Antibodies may be formed against erythrocyte antigens (type II hypersensitivity), against a non-erythrocyte antigen attached to the red cell surface (type III hypersensitivity), or an antibody may be produced against an unassociated antigen that is similar to an erythrocyte antigen. Alloantibodies present in transfused blood or in colostrum from a type B queen ingested by a type A kitten may lead to immune-mediated hemolysis. Some erythrocyte antigens are hidden and exposed to the immune system only after the cell membrane has been damaged. New antigens that cross-react with red cell antigens or attach to the red cell membrane may be released into circulation by infection or inflammation.34
The antibodies involved in the immune process are usually IgG, although IgM can be present alone or with IgG. Macrophages of the mononuclear phagocyte system have receptors for the Fc portion of the IgG antibody but not for IgM. Fc receptors are proteins on the surfaces of cells such as macrophages and neutrophils that contribute to the protective functions of the immune system. Fc receptors bind to the Fc portion of antibodies attached to pathogens or infected cells and stimulate the activity of phagocytic or cytotoxic cells. The antibody-coated cells are removed after antibody binds to the receptor on the macrophage, mostly in the red pulp of the spleen. The result is extravascular hemolysis. Complete phagocytosis may not occur, and only a portion of the membrane may be removed. The cell’s volume-to-surface-area ratio is reduced, and the cell becomes spherical. Spherocytes are less able to deform, making passage through the spleen more difficult. Because of the nonsinusoidal nature of the feline spleen (see the section on splenic diseases later in this chapter), less cell deformability is required for cells to pass through, and decreased numbers of spherocytes are trapped and destroyed than in dogs. Splenic macrophages also have receptors for complement. If a sufficient quantity of IgG antibodies coat the cell membrane, complement may also bind to the erythrocyte. The presence of complement on the cell membrane increases the efficiency of erythrocyte removal. If a sufficient number of the antibodies are IgM, complement-mediated lysis can occur, resulting in intravascular hemolysis. However, none of the 19 cats with primary IMHA in one study had intravascular hemolysis despite the presence of IgM in 8 of the cats.57
Cats with IMHA, whether primary or secondary, will exhibit signs related to the anemia. These can include anorexia, lethargy, weakness, or respiratory difficulties. Additional signs as a result of the underlying disease may be present in cats with secondary IMHA. Most of the cats with primary IMHA are young adults. In the previously mentioned report of 19 cats with primary disease, six were younger than 2 years old; the median age for all 19 cats was 2 years.57 Eleven of the cats were male, and eight female. Cats with secondary disease will have a signalment related to the underlying disease. Physical changes that might be present include pale or icteric mucous membranes, tachycardia, tachypnea, or splenomegaly as a consequence of increased processing of damaged erythrocytes. Tachypnea and tachycardia are attempts at compensating for the decreased oxygen-carrying capacity of the anemic patient; pulmonary thromboembolism, so common in dogs with IMHA, is rare in cats.57 Splenomegaly occurs in many nonimmune disorders causing hemolysis as the organ attempts to deal with the increased numbers of damaged red cells. Body temperature is likely to be normal unless the patient is moribund, in which case hypothermia may be noted. A systolic murmur may be heard during auscultation of the thorax.
Diagnosis of IMHA can be frustrating. There are many mechanisms causing hemolysis that do not involve the immune system. Distinguishing primary from secondary IMHA is important because therapy may be different. With aggressive diagnostic investigation, an underlying cause is often found. Two sources state that primary IMHA is rare in cats.34,75 However, Kohn and coworkers57 found that of 23 anemic cats with a positive Coombs’ test or persistent erythrocyte agglutination, an underlying cause was identified in only four patients after an extensive workup. Put another way, 19 of the 23 Coombs’-positive cats had primary IMHA.
If the hemolysis is severe enough and the anemia is not peracute, there should be evidence of regeneration in the CBC report. Increased numbers of aggregate reticulocytes should be present. Polychromasia and rubricytosis (nucleated red blood cells) may be present. The hallmark of IMHA in dogs, spherocytosis, is unlikely to be identified. The PCV may be surprisingly low for the condition of the patient; cats seem to tolerate a lower PCV than dogs.57 The lower the PCV, the higher the aggregate reticulocyte count should be. If the reticulocyte count is not appropriate for the degree of anemia, it may be nonregenerative. In this case an immune response directed at erythrocyte precursors in the bone marrow might be considered. Another difference from dogs is the lack of leukocytosis or neutrophilia with left shift in cats with primary IMHA. In the aforementioned study by Kohn and coworkers57 17 of the 19 cats with primary IMHA had a white cell count within the reference range. The platelet count should be within the reference range, too. Evans syndrome, a combination of immune-mediated damage to both erythrocytes and platelets, is rare in cats. Although evidence of DIC is common in dogs with IMHA, it is uncommon in cats. Before convicting a cat with anemia of also having thrombocytopenia on the basis of an automated cell count, a blood smear should be examined to determine whether platelet clumping is present. The smear examination will also allow identification of intraerythrocytic parasites or morphologic changes in the erythrocyte that may suggest causes of anemia other than IMHA.
The direct Coombs’ test detects the presence of antibodies or complement on the red cell surface. A positive test is consistent with, but not necessarily diagnostic for, IMHA. However, a diagnosis of IMHA should include a positive direct Coombs’ test.34 False-negative tests are unlikely. In the study by Kohn and coworkers, 78 cats with anemia had a direct Coombs’ test performed and 55 were negative, all of which had nonimmune etiologies identified as causing the anemia; an additional 14 cats without anemia were also direct Coombs’ test negative.57 The direct Coombs’ test may become negative after a patient with IMHA enters remission, although a few days of immunosuppressive therapy is unlikely to cause a negative test.34 A limitation of the test is the inability to differentiate between primary and secondary IMHA. A properly performed slide agglutination test may detect anti-erythrocyte IgM or large quantities of anti-erythrocyte IgG coating the erythrocytes. Autoagglutination must be distinguished from rouleaux formation by proper washing or dilution of the blood on the slide. A direct Coombs’ test is unnecessary if the slide agglutination test is positive because they both test for anti-erythrocyte antibodies; autoagglutination is considered indicative of IMHA.57 Autoagglutination may artifactually increase the MCV because clumps of cells are counted as one.
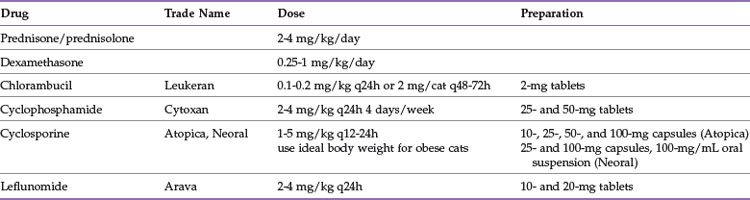
Reduction of the immune-mediated destruction of erythrocytes is the goal of drug therapy. The optimal drug protocol will decrease phagocytosis of antibody or complement coated red cells, reduce complement activation, and eliminate the production of anti-erythrocyte antibodies (Table 25-3). Glucocorticoids are the initial drug of choice. These drugs are both antiinflammatory and immunosuppressive, although higher doses are required to accomplish the latter. Oral prednisone is the most commonly used glucocorticoid, but it requires conversion by the liver to the active form, prednisolone.100 There is some evidence that intestinal absorption or hepatic conversion of prednisone to prednisolone may be poor in cats,119 thus prednisolone is thought by some to be a better initial choice in this species. The pharmacologic effects are due to interference with cellular communication and interaction among cells of the immune system. Glucocorticoids also inhibit production of cytokines used to amplify the immune response.75 Decreased production of IL-2 leads to decreased Th1 helper cell proliferation and cytotoxicity.27 Glucocorticoids stimulate maturation of T-suppressor cells and inhibit antibody-dependent cytotoxicity by natural killer cells,27 resulting in inhibition of the cellular arm of the immune system. They are beneficial in reducing binding of the Fc component of the attached IgG to the Fc receptors on splenic macrophages. In addition, they may decrease antibody binding to the red cell membrane and complement activation.34 There are few direct effects on B-lymphocytes, and therefore there is little effect on antibody production.27,90 Cats have fewer and less sensitive cytoplasmic glucocorticoid receptors than dogs.16 This may explain why cats typically have less pronounced side effects; for instance, polyuria and polydipsia and steroid hepatopathy are not typical side effects of glucocorticoid use in cats.27 Cats receiving immunosuppressive doses of glucocorticoids may have difficulty eliminating infections on their own, and some infections may be inapparent because the inflammation associated with the infection may be blunted by the antiinflammatory effect of the glucocorticoid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 inches), stylet for the Rosenthal needle.
inches), stylet for the Rosenthal needle.