CHAPTER 7 Anesthesia and Perioperative Care
Assessment of Risk
In both medical and veterinary anesthesia, patients are often classified using the American Society of Anesthesiologists Physical Status Classification (ASA-PS), which attempts to give a subjective and relative risk based only on the patient’s preoperative medical history (Table 7-1). In this classification ASA 1 is considered a healthy patient with no overt signs of disease, and 5 is considered a moribund patient who is considered likely to die in the next 24 hours with or without surgery. Addition of “E” to the classification indicates emergency surgery.61
TABLE 7-1 American Society of Anesthesiologists’ Physical Status Classification
| Class* | Preoperative Health Status | Comments |
|---|---|---|
| PS 1 | Normal healthy patient | No health problems; excludes the very young and very old |
| PS 2 | Patients with mild systemic disease | Mild, well-controlled systemic disease |
| PS 3 | Patients with severe systemic disease | Severe or poorly controlled systemic disease |
| PS 4 | Patients with severe systemic disease that is a threat to life | At least one disease that is poorly controlled or end stage, possible risk of death |
| PS 5 | Moribund patients not expected to live >24 hours with or without surgery | Imminent risk of death, multiorgan failure |
* An E is added to the class to designate emergency surgery.
Adapted from http://www.asahq.org/clinical/physicalstatus.htm.
Although anesthetic-related death in cats has decreased over the years, the most recent published mortality rate of 0.24%, or 1 in 453 anesthetics,27 is still up to 10 times that found in human studies.42 The “Confidential Enquiry into Perioperative Small Animal Fatalities”26 was undertaken in 117 veterinary practices in the United Kingdom from 2002 to 2004. The study included 79,178 cats with overall risks of sedation and anesthetic-related deaths within 48 hours of procedure of 0.24%. In this study most cats were premedicated (70%), intubated (70%), and breathing spontaneously (92%). Procedures were short (25 to 30 minutes), and fluids were administered to only 26% of cats. Monitoring was rare, with pulse monitored in 38%, pulse oximetry in 16%, and both pulse and pulse oximetry in 25% of cats. Temperature was monitored intraoperatively in 1% to 2% of cats and postoperatively in 11% to 15% of cats. Specifically in cats, factors associated with increased odds of anesthetic-related death were poor health status (ASA-PS classification), increasing age, extremes of weight, increasing procedural urgency and complexity, endotracheal intubation, and fluid therapy. In this study the greater risk associated with anesthesia in cats compared with dogs was reported to be related to their size (relatively small with a large surface area to volume ratio), which predisposes them to hypothermia and drug overdosage, and a small airway and a sensitive larynx, which predisposes them to upper airway complications. Pulse monitoring and pulse oximetry were associated with reduced odds, related more to patient monitoring than to the specific equipment used. A total of 61% of cats died in the postoperative period, with 62% of those occurring in the first 3 hours after surgery. Factors considered important in reducing mortality risk are listed in Box 7-1.
Sedation and Premedication
• Sedation to facilitate intravenous catheterization and induction of anesthesia
• Reduction of stress and anxiety
• Reduction of anesthetic dose for induction and maintenance to reduce adverse effects due to anesthetic agents
• Prevention or treatment of adverse effects of other drugs given for premedication
• Anesthetic induction, or maintenance
• Improvement in quality of anesthetic induction and/or recovery
Agents commonly used for premedication belong to one of three classes: tranquilizers/sedatives, analgesics, and anticholinergics. The pharmacology of drugs commonly used for premedication is briefly reviewed in Table 7-2.
TABLE 7-2 Drugs Commonly Used for Sedation and Premedication in the Cat
| Drug | Main Desired Effect | Suggested Dose Range and Route |
|---|---|---|
| Acepromazine | Sedation | 0.02-0.05 mg/kg SC, IM, IV |
| Diazepam | Sedation | 0.1-0.5 mg/kg IV |
| Midazolam | Sedation | 0.1-0.3 mg/kg IM, IV |
| Xylazine | Sedation | 0.5-2 mg/kg SC, IM, IV |
| Dexmedetomidine | Sedation | 5-20 µg/kg SC, IM, IV |
| Morphine | Analgesia | 0.1-0.2 mg/kg SC, IM |
| Hydromorphone | Analgesia | 0.03-0.1 mg/kg SC, IM, IV |
| Oxymorphone | Analgesia | 0.03-0.1 mg/kg SC, IM, IV |
| Methadone | Analgesia | 0.2-0.5 mg/kg SC, IM, IV |
| Buprenorphine | Analgesia | 10-30 µg/kg SC, IM, IV |
| Butorphanol | Analgesia | 0.1-0.4 mg/kg SC, IM, IV |
| Ketamine | Sedation | 5 mg/kg SC, IM; 2-5 mg/kg IV |
| Telazol | Sedation | 3-5 mg/kg SC, IM; 2-3 mg/kg IV |
| Atropine | Prevention of bradycardia, decreased secretions | 0.01-0.04 mg/kg SC, IM, IV |
| Glycopyrrolate | Prevention of bradycardia, decreased secretions | 0.01 mg/kg SC, IM, IV |
SC, Subcutaneous; IM, intramuscular; IV, intravenous.
Tranquilizers and Sedatives
Acepromazine
Acepromazine is the prototype tranquilizer and is the only drug in that category commonly used in clinical practice (Box 7-2). Acepromazine is a phenothiazine compound. It antagonizes the actions of dopamine as a central neurotransmitter. It also blocks the effects of dopamine at peripheral D1 and D2 receptors. Its onset of action is long (15 minutes after intravenous administration, 30 to 45 minutes after intramuscular administration), and it has a long (3 to 6 hours) duration. Acepromazine is sometimes administered orally, but its bioavailability appears poor,88 although data in cats are not available. High doses should therefore be used.
Acepromazine produces sedation. Typically, patients are rousable by stimuli of sufficient intensity. The sedative effect is variable among individuals but may be improved by combining acepromazine and opioids (neuroleptanalgesia). Chlorpromazine, another phenothiazine, was shown to decrease morphine-induced excitement in cats,48 and acepromazine is expected to have similar effects. Phenothiazines appear to suppress aggressive behaviors related to dominance rather than fear. Acepromazine is usually not thought to produce analgesia. However, in a recent study in cats, acepromazine produced mechanical antinociception and potentiated the effect of tramadol.211 Acepromazine has been reported to decrease anesthetic requirements, both for injectable and inhaled anesthetics.95,233 In a study in cats, however, acepromazine did not reduce the induction dose of propofol.69 Phenothiazines may decrease the seizure threshold,57,128 and acepromazine should be used with caution in patients with a history of seizures or during procedures or with drugs that may cause seizures.
Acepromazine produces minimal effects on the respiratory system. Respiratory rate may decrease, but blood gases remain normal, probably because of an increase in tidal volume. Acepromazine produces vasodilation and hypotension.41 The effect is mainly due to alpha-adrenergic blockade; central sympatholysis, direct vasodilation, and/or stimulation of beta2 adrenergic receptors may contribute. If a vasoconstrictor is used to treat hypotension in cats receiving acepromazine, an alpha1 agonist devoid of beta2 effect such as phenylephrine or norepinephrine should be used. Heart rate may decrease, but the effect is usually mild. Phenothiazines protect against epinephrine-induced arrhythmias.153 They cause splenic sequestration of red blood cells and markedly reduce the hematocrit level.
Acepromazine interferes with temperature regulation. Hypothermia or hyperthermia may result, depending on ambient temperature, although hypothermia is more common. Acepromazine produces antiemetic effects because of its interaction with central dopaminergic receptors at the level of the chemoreceptor trigger zone. Acepromazine reduces gastroesophageal sphincter pressure, possibly increasing the incidence of esophageal reflux and regurgitation.90 Acepromazine blocks histamine H1 receptors and may affect the results of intradermal skin testing.14 Acepromazine applied topically does not affect intraocular pressure in normal eyes but may reduce it when elevated.94 Acepromazine reduces tear production in normal cats.70
Benzodiazepines
Three drugs in the benzodiazepine class are used in clinical practice as part of anesthetic management: diazepam, midazolam, and zolazepam. Zolazepam is available only in combination with tiletamine (Telazol) and will not be discussed here (Box 7-3).
Clinical effects relevant to anesthesia include sedation or dysphoria, decreased anxiety, inhibition of aggressive behavior, amnesia, muscle relaxation, anticonvulsant effects, and reduced anesthetic requirements. Benzodiazepines do not appear to produce analgesia after systemic administration. In cats 1 mg/kg of diazepam administered intramuscularly caused apparent sedation; however, when cats were restrained for handling, they vigorously objected.93 A study examined the effects of midazolam, administered intravenously or intramuscularly, at various doses ranging from 0.05 to 5 mg/kg.108 Restlessness was observed initially, followed by sedation, with most cats receiving the higher doses intravenously assuming a lateral recumbency. When cats were restrained, an approximately equal proportion responded more and less than normal, independent of dose and time. It therefore appears that benzodiazepines do not consistently produce sedation in cats, at least when administered alone. Combinations with opioids may improve the consistency of the sedative effect.
Benzodiazepines are commonly used with induction agents to improve muscle relaxation and/or reduce the anesthetic dose. Diazepam and midazolam have been reported to decrease the anesthetic dose of both inhaled and injectable anesthetics.* They are very effective at preventing and treating convulsions. In humans midazolam is useful in the treatment of status epilepticus refractory to phenobarbital, phenytoin, and diazepam.227
Benzodiazepines produce minimal cardiovascular and respiratory effects. Diazepam may decrease ventricular arrhythmias resulting from myocardial ischemia.152 In hypovolemic patients high doses of midazolam may produce hypotension.3 Hypotension, arrhythmias, and asystole have been reported after intravenous administration of diazepam; this is thought to be due to propylene glycol, which is used as a solvent in commercially available solutions.79
The main difference between diazepam and midazolam is related to their physicochemical characteristics and pharmacokinetics. Diazepam is highly hydrophobic, and studies in humans suggest that absorption may be poor after administration in some muscle groups. Midazolam is hydrophilic at low pH and lipophilic at higher pH; it may be better suited to intramuscular administration than diazepam. Its bioavailability after intramuscular administration is higher than 90% in humans and dogs. Onset of effect is short for both drugs. Diazepam undergoes oxidation to nordiazepam, an active metabolite, which is eliminated about 6 times more slowly than diazepam. The clearance of diazepam itself in cats is low. Diazepam is therefore expected to have long-lasting effects.43 There are no published data on the pharmacokinetics of midazolam in cats. However, in dogs midazolam is rapidly eliminated, in contrast to diazepam.44,126 In the species in which it has been examined, the metabolism of midazolam results in the production of hydroxymidazolams, which have pharmacologic activity but are usually rapidly eliminated. Clinically, the duration of effect of midazolam appears much shorter than that of diazepam.
Acute fulminant hepatic necrosis has been reported in cats following diazepam administration.34 However, it followed repeated oral administration; similar toxicity has not been reported after occasional parenteral administration of the drug.
Alpha2-Adrenoceptor Agonists
Agonists of the alpha2-adrenergic receptors (alpha2 agonists) act mainly by modulating noradrenergic transmission in the central nervous system. They also have direct effects on various organs. Drugs in this class commonly used in cats include xylazine and dexmedetomidine (Box 7-4).
Alpha2 agonists produce sedation; the effect is dose dependent.214 At high doses sedation is profound, and patients are unresponsive to most stimuli, although arousal and aggressive behavior is always possible. Alpha2 agonists also produce analgesia.228 The duration of the analgesic effect of both xylazine and dexmedetomidine appears short.154,208 Alpha2 agonists reduce anesthetic requirements in a dose-dependent manner. They induce hypothermia through an effect of the hypothalamic thermoregulatory center.
Respiratory effects produced by alpha2 agonists are considered minimal in cats. Respiratory rate tends to decrease, but blood gases are usually unaffected.78,117
The typical cardiovascular response to the administration of alpha2 agonists is biphasic. Initially, blood pressure and systemic vascular resistance increase, whereas heart rate and cardiac output decrease.74,117,154 The increase in blood pressure may not be seen after intramuscular administration. These effects are followed by a decrease in arterial pressure; heart rate and cardiac output remain lower than normal. Systemic vascular resistance either returns progressively toward normal or remains elevated, depending on the drug and the dose considered. The bradycardia may be accompanied by other arrhythmias. The cardiovascular effects of alpha2 agonists are usually considered to be dose dependent. The increase in systemic vascular resistance is due to stimulation of alpha2 receptors on the vascular smooth muscle, resulting in vasoconstriction. The decrease in cardiac output is due to the decrease in heart rate. Myocardial contractility appears unaffected.
Because the decrease in cardiac output appears to be mainly related to the bradycardia, the combination with anticholinergics has been advocated. However, the concomitant use of anticholinergics with alpha2 agonists is controversial. The effectiveness in increasing heart rate could depend on the timing of administration of the drugs. When given before the alpha2 agonist, anticholinergics tend to increase heart rate, which decreases after the alpha2 agonist is administered. When given simultaneously, there is an initial bradycardia followed by a return of heart rate toward baseline values. In both cases severe hypertension is produced, and cardiac performance further decreases.6,50,206
Clinically, xylazine and dexmedetomidine are used mainly for their sedative effect. They are sometimes used to improve analgesia. Combinations with opioids may reduce the dose required to produce sedation.199 Because of their cardiovascular effects, they should be used with caution in geriatric patients or patients with significant organ dysfunction. The use of medetomidine in cats with hypertrophic cardiomyopathy and left ventricular outflow tract obstruction has been suggested to decrease the obstruction; dexmedetomidine is expected to produce similar effects.118
Dissociative Anesthetics
Ketamine and Telazol are sometimes used as premedication before general anesthesia. Their pharmacology is reviewed in the section on induction agents. Dissociative anesthetics produce dose-dependent effects ranging from mild or moderate sedation to anesthesia. They may be useful in the intractable cat, as long as an injection can be administered. Ketamine should not be used alone because of its effect on muscle tone and the risk for convulsions; it should be combined with acepromazine, a benzodiazepine, or an alpha2 agonist (Box 7-5).
Opioids
The pharmacology of opioids is reviewed in Chapter 6. Only their use in the context of premedication will be addressed here.
Opioids are used for their analgesic effect (Box 7-6). They are commonly given at the time of premedication to produce preemptive analgesia. Because they are considered to be the first line of treatment for acute (surgical) pain, they should be included in the anesthetic regimen for any procedure likely to cause pain. In addition to their analgesic effect, they reduce the effective dose of sedative and anesthetic drugs. They also produce some behavioral modification. Usually, at the doses recommended for clinical use, opioids produce euphoria in cats (i.e., cats do not appear sedated but are more playful and resist restraint less). At higher doses dysphoria may be produced, and cats become hyperactive, excitable, and more difficult to handle. Various drugs can be used. Typically, the full agonists (e.g., morphine, hydromorphone, oxymorphone, methadone) are considered to have a higher analgesic efficacy than the partial agonist buprenorphine. The agonists–antagonists such as butorphanol usually have low analgesic efficacy. However, buprenorphine, at the doses commonly used clinically, appears to produce good analgesia in cats.
Anticholinergics
Anticholinergics antagonize the effects of acetylcholine at muscarinic receptors, which result in the blockade of transmission at parasympathetic postganglionic nerve terminals. They decrease overall parasympathetic tone (Box 7-7).
Induction Agents
Toxicity studies in cats allowed the therapeutic index of ketamine, thiopental, and alphaxalone to be derived.36 In one study36 the difference between the dose that caused recumbency and the fatal dose was 4 times for thiopental and 5 times for ketamine and alphaxalone.
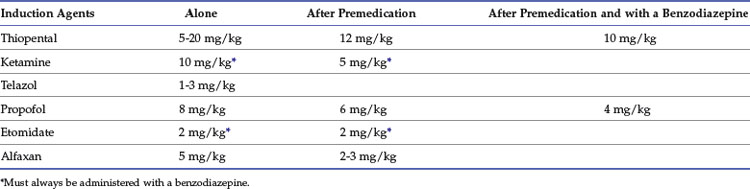
Calculated intravenous induction doses, reported in Table 7-3, vary depending on the end point and whether the agent is administered after premedication or in conjunction with a benzodiazepine.
Thiopental
Thiopental is the oldest of the injectable anesthetic agents, having been introduced into veterinary practice in the early 1930s. It is a rapidly acting thiobarbiturate with an ultrashort duration of action. It is marketed as the sodium salt in powder form and is reconstituted with 0.9% sodium chloride or water for injection. The usual concentration for clinical use is 2.5%. The drug is a weak acid, and because the unionized form is poorly water soluble, concentrated solutions for administration are alkalinized so that the drug is restricted almost entirely to the water-soluble ionized form. The high pH of the solution is partly responsible for the irritancy of the drug if it is given perivascularly (Box 7-8).
BOX 7-8 Advantages and Disadvantages of Thiopental
Advantages
• It is a rapidly acting drug, with effects discernible within a circulation time. Thiopental lends itself to titration to effect and is especially useful when an airway needs to be secured quickly, such as in a cat with a full stomach or a history of vomiting.
• It has an ultrashort duration of action (5-10 minutes) depending on administered dose. Thiopental is an excellent induction agent before intubation and maintenance with inhalant agents. It is also suitable for nonpainful procedures of short duration (15-20 minutes), although propofol provides better recovery conditions.
• It decreases intracranial pressure (ICP) in patients with raised ICP and has protective cerebral effects if administered before a hypoxemic event. It is an effective anticonvulsant, although its anesthetic and anticonvulsant effects cannot be separated.
• It depresses laryngeal reflexes less than other induction agents, such as propofol and ketamine, and therefore facilitates examination of vocal cords and correct diagnosis of laryngeal paralysis.
Disadvantages
• It is not a suitable drug for maintenance of anesthesia because clearance is slow, leading to accumulation and prolonged recoveries.
• It is an irritant if given perivascularly, and treatment is important to prevent tissue necrosis and sloughing.
• It decreases packed cell volume and white blood cell and platelet counts and may decrease total protein concentration.
• It does not block autonomic responses to noxious stimuli and thus is not suitable for short painful procedures.
• Recovery can be rough, especially if the patient awakes from thiopental alone.
• It is a myocardial-depressant drug that induces tachycardia and an increased incidence of arrhythmias. In healthy animals these arrhythmias are rarely of clinical importance.
• Laryngeal reflexes are active, increasing the difficulty of intubation. Because of this, traumatic intubation may be more likely with thiopental than other agents.
Pharmacodynamic Effects
Although thiopental has been used in veterinary anesthesia for many years, there are few reports concerning pharmacologic effects in cats. Early cardiopulmonary studies reported a decrease in respiratory rate and tidal volume, a fall in blood pressure, and a slowing of heart rate.80 A dose of 20 mg/kg produced mild hypotension between 5 and 10 minutes after administration, with little change in heart rate. Approximately 30% of cats developed apnea lasting up to almost 1 minute, and arterial carbon dioxide tension was elevated and arterial oxygen tension decreased at 1.5 minutes.143 A more in-depth study undertaken after acepromazine (0.2 mg/kg), meperidine (4 mg/kg), and atropine (0.05 mg/kg) premedication followed by induction with thiopental (10 mg/kg) reported minor respiratory depression but a significant fall in cardiac index with no change in heart rate.51 Thiopental (6.8 mg/kg) had a negative effect on the cat myocardium, suggesting that the hypotension may be due to direct myocardial depression.76
Thiopental induces a dose-related depression of cerebral metabolic oxygen consumption rate and, presumably because of preserved cerebral autoregulation, reduces cerebral blood flow.142 As a result of the reduced cerebral blood flow and accompanying fall in cerebral blood volume, cerebrospinal fluid pressure is reduced. With thiopental, as with etomidate, cerebral perfusion pressure is not compromised because intracranial pressure decreases more than mean arterial pressure. Thiopental is an effective anticonvulsant, although its hypnotic and anticonvulsant properties occur at similar doses.
No difference in the incidence of gastroesophageal reflux was reported in cats between thiopental and propofol, with an incidence of 16% and 12%, respectively.66
Pharmacokinetic Effects
The pharmacokinetics of thiopental in cats has not been reported, although that of a very similar thiobarbiturate, thiamylal, has been described.238 The rapid distribution half-life was 1.91 minutes, and a second, or slower, distribution half-life was 26.51 minutes. The elimination half-life was 14.34 hours. The apparent volume of distribution was 3.61 L/kg, whereas the apparent volume of the central compartment was 0.46 L/kg, and the total clearance was 0.135 L/kg/h. As in other species, initial wake-up is due to redistribution initially into vessel-rich tissues and muscle and later into fat.28,29 Although the drug does not have a high clearance, metabolism does contribute to recovery.192
Ketamine
Ketamine is partly water soluble and is prepared in a slightly acidic (pH 3.5 to 5.5) solution. It is formulated for veterinary use as a 10% solution in sodium chloride with the preservative, benzethonium chloride (Box 7-9).
BOX 7-9 Advantages and Disadvantages of Ketamine
Advantages
• Rapidly acting agent without excitement and with a duration of action that allows induction of anesthesia to proceed slowly
• Excellent analgesic agent even at subanesthetic doses with demonstrated preemptive analgesic properties
• Reported to induce less respiratory depression than thiopental or propofol, and respiratory responses to hypoxemia and hypercarbia are better maintained
• Potent bronchodilating drug that is a suitable induction agent in asthmatic patients or patients with reactive airways
Disadvantages
• It increases muscle tone and induces purposeless muscle movements, making it difficult to carry out certain procedures.
• Salivation and lacrimation are present and may be profuse.
• Intraocular pressure may increase, and eyes remain open and are susceptible to corneal abrasion.
• Cerebral blood flow and cerebral oxygen consumption increase, which may have serious adverse effects in patients with raised intracranial pressure.
• Ketamine depresses the myocardium, but central sympathetic stimulation leads to an increase in heart rate, blood pressure, and cardiac output. In diseases such as hypertrophic cardiomyopathy, these cardiovascular effects may prove fatal.
Ketamine is considered a dissociative anesthetic, a term used to describe a state in which there is functional and electrophysiologic dissociation between the thalamoneocortical and limbic systems.240 This unique clinical state of hypnosis and analgesia is characterized by open eyes, dilated pupils, muscle hypertonus, and increased lacrimation and salivation. An anticholinergic drug, atropine or glycopyrrolate, is usually administered as a preanesthetic to decrease salivation.
Ketamine acts primarily through the N-methyl-D-aspartate (NMDA) receptor.112,158
Clinical Use
Induction of anesthesia with ketamine alone is unsatisfactory insofar as muscle tone is extreme and spontaneous movement virtually continuous.246 Tranquilizers are often administered before ketamine, whereas the benzodiazepines, diazepam or midazolam, are usually administered in combination with ketamine to eliminate or minimize the deleterious side effects. When ketamine is used as an induction agent, the calculated dose in unpremedicated healthy cats is 10 mg/kg IV,86 together with diazepam (0.5 mg/kg IV) or midazolam (0.3 to 0.5 mg/kg IV). Premedication reduces the dose of ketamine to 5 mg/kg, and the dose of diazepam or midazolam remains the same. Studies in cats using a calculated dose of 3 mg/kg of ketamine reported that the ED50 (effective dose in 50% of the population) of midazolam required for intubation and to prevent movement in response to a noxious stimulation was 0.286 mg/kg and 0.265 mg/kg, respectively. At that dose recovery to walking with ataxia took 41.50 ± 15.18 minutes and complete recovery 3.6 ± 1.3 hours.107
One quarter of the calculated dose of ketamine, followed by one half of the calculated dose of diazepam or midazolam, is usually administered over 10 to 20 seconds and the patient observed for drug effects after 1 minute. If more of the drug is required, another quarter of the calculated dose of ketamine and the remainder of the diazepam or midazolam is administered over another minute. Some veterinarians prefer to combine the drugs in the same syringe and then just administer in quarter-dose boluses.86
Pharmacodynamic Effects
Ketamine is reported to have sympathomimetic effects, which increase heart rate, cardiac output, and blood pressure, primarily by direct stimulation of central nervous system structures.240 In the absence of autonomic control, ketamine has direct myocardial depressant properties.223,245 The cardiopulmonary effects of intravenous administration of ketamine have been studied in cats.143 At clinical doses (6.6 mg/kg intravenously), both heart rate and blood pressure increased, with peak effects generally occurring 2.5 minutes after administration. Transient respiratory depression was also reported.143 In cats anesthetized with halothane, ketamine has been reported to decrease the arrhythmogenic threshold.17 Respiration has been noted as apneustic, shallow, and irregular immediately after ketamine administration in cats.37
Ketamine has bronchodilating properties167 and is often recommended as the induction agent of choice in cats with asthma.
Swallow, cough, and gag reflexes are relatively intact after ketamine, and in cats, but not humans, competent laryngeal protective reflexes are maintained, such that material that reaches the trachea is coughed up and swallowed.188,221 In cats contrast radiography has been suggested as a diagnostic aid in ketamine-anesthetized cats suspected of laryngeal reflex abnormalities.188
Care is needed in interpretation of echocardiographic measurements in cats under light sedation doses (1.5 to 2.5 mg/kg intravenously) of ketamine, insofar as significant differences were reported in studies conducted using different drugs or in non-anesthetized cats.64
Although there are no specific published studies in cats, in other species ketamine increases cerebral blood flow and intracranial pressure, principally by cerebral vasodilation and elevated systemic arterial pressure.200,218 Part of the vasodilation is due to increased arterial carbon dioxide tensions when ventilation is not controlled,196 and the other most likely results from stimulation of cerebral metabolic rate. Thus the use of ketamine in patients with raised intracranial pressure is not recommended. Ketamine has been reported to cause seizures in cats,15,186 although usually only after intramuscular administration of high doses.
Under ketamine anesthesia, segmental stretch and withdrawal reflexes are preserved even at levels that block electroencephalographic arousal and autonomic changes in response to nociceptive stimulation.220
Ketamine, compared with thiopental, propofol, and Saffan, had the least effect on gastroesophageal sphincter pressure and barrier pressure in cats.89
Administration of ketamine interferes with the results of glucose tolerance tests in cats and thus this test should be performed without chemical restraint.102
In cats a slight but significant increase in intraocular pressure occurs with ketamine,82 so this agent should be avoided if the cat is at risk for corneal perforation. The increase is thought to be due to increases in extraocular muscle tone induced by ketamine.
Cats induced with ketamine (5 mg/kg intravenously) and diazepam (0.25 mg/kg intravenously) and maintained with halothane anesthesia were found to mount a normal response to an ACTH stimulation test, indicating adequate adrenocortical function.147
Various sedative protocols, including those containing ketamine, have been reported to produce significant effects on thyroid function and salivary gland uptake of technetium Tc 99m pertechnetate and, as such, may interfere with thyroid scintigraphic image interpretation.194
Sedation with ketamine results in a lower number of spermatozoa per ejaculate compared with medetomidine when semen was collected after electroejaculation.249
Ketamine has been reported to possess analgesic properties. Induction with ketamine or addition of ketamine to general anesthesia before surgical stimulation decreases postoperative pain and leads to better pain control.156,191 It appears that the analgesic properties of ketamine reduce sensitization of pain pathways and extend into the postoperative period. Although ketamine appears to provide good somatic analgesia, its visceral analgesia is weak.193
Pharmacokinetic Effects
Recovery from ketamine is due to both redistribution and metabolism. Several studies have reported the pharmacokinetic profile of ketamine in cats.10,96 Ketamine has a rapid distribution with a brief distribution half-life of 5.2 minutes. The high lipid solubility is reflected in the large volume of distribution (3.21 L/kg). Clearance is also high (37.8 mL/kg/min), which accounts for the short elimination half-life (60.6 min).96 Mean total body clearance is very similar to liver blood flow, which means that changes in liver blood flow affect clearance. This has been reported in the cat, where xylazine prolonged the duration of ketamine anesthesia by increasing the elimination half-life.232 Ketamine is metabolized extensively in the liver to form norketamine (metabolite I), which has 20% to 30% of the activity of the parent drug. Cats, as a species, are unable to metabolize norketamine further, and elimination of norketamine is dependent on renal excretion. Thus duration of action may be prolonged in cats with severe renal dysfunction.
Telazol
Clinical Use
Although Telazol is not registered for intravenous use in cats, it is a suitable induction agent. The reported dose is 1 to 3 mg/kg, and administration of an anticholinergic is recommended because salivation can be profuse.129 A dose of 3 mg/kg is diluted to 1 mL with saline, and a 1 mg/kg bolus is administered intravenously every 1 minute until intubation is possible.
A dose of 9.9 mg/kg administered intravenously or intramuscularly resulted in a similar duration of anesthesia (20 minutes) and time to walking (174 to 180 minutes).224
Pharmacodynamic Effects
There is little published data on effects of Telazol, especially in cats, and it is presumed that the effects are similar to ketamine.125,129 The cardiovascular and respiratory effects of intravenous administration of Telazol have been reported, although the doses (9.7, 15.8, and 23.7 mg/kg) were higher than those commonly used in practice.98 An initial cardiovascular depressor response, with degree and duration depending on the dose, was then followed by a pressor response. In another study Telazol did not alter the arrhythmogenic threshold in cats.16
The degree of respiratory depression in cats appears to be dose dependent, with higher doses causing more depression. In one study respiratory rate decreased and was often characterized initially by an apneustic respiratory pattern.248 Within 10 to 15 minutes, respiration had returned to a normal pattern.224 Periods of apnea have been reported after intravenous administration of high doses (15.8 and 23.7 mg/kg), and arterial carbon dioxide tension was elevated.98
Telazol is considered a suitable intravenous agent for intradermal skin testing in cats.149
Telazol carries the same warning as other dissociative agents.129 It is not recommended for cats with hypertrophic cardiac disease, hepatic or renal disease may prolong the actions, and dose-dependent respiratory depression is reported when Telazol is administered intravenously with other anesthetic drugs. According to further information on the package insert, use in animals with severe cardiac or pulmonary dysfunction and those requiring cesarean section is not recommended.
Pharmacokinetic Effects
The plasma half-life of tiletamine in cats was reported as 2 to 4 hours, with only 5% to 10% of the dose detected in urine, none in feces, and some in bile. Three metabolites were detected in the urine from cats.125 The plasma half-life of zolazepam in cats was reported as 4.5 hours, with three metabolites detected in urine.125
Propofol
Propofol is a substituted isopropylphenol, which is only slightly soluble in water and is formulated as a 1% aqueous solution containing soybean oil, egg lecithin, and glycerol. It has a rapid onset, with a smooth, excitement-free induction (Box 7-10).
BOX 7-10 Advantages and Disadvantages of Propofol
Advantages
• Rapidly acting drug with minimal excitement, even after subanesthetic doses.
• Recovery is rapid, smooth, and complete, making it an ideal outpatient anesthetic.
• It can be used to induce anesthesia before intubation, or anesthesia can be maintained with propofol by either repeated bolus injections or constant-rate infusion.
• It decreases intracranial pressure in patients with raised intracranial pressure and has protective cerebral effects if administered before a hypoxemic event.
• It decreases intraocular pressure and is a good induction agent in cats with descemetoceles or corneal lacerations.
• It is the induction agent of choice in healthy queens requiring cesarean section if viability of the kittens is important.
• It induces bronchodilation and is a suitable agent in asthmatic patients.
Disadvantages
• Apnea is the most common side effect in cats, and cyanosis is often observed during induction.
• Myoclonus sometimes occurs on induction and, if severe, may prevent surgery.
• It has myocardial-depressant and vasodilatory properties without altering heart rate and may cause hypotension, especially in hypovolemic and geriatric patients.
• Bacterial contamination of the solution can increase the incidence of surgical wound infection or cause sepsis.
• Autonomic responses to noxious stimuli are not blocked, and therefore it is not a suitable anesthetic agent for painful procedures.
• Cats have reduced capacity to conjugate propofol, and length of recovery increases with increasing duration of propofol anesthesia.
• Care should be taken if propofol is administered to cats over consecutive days because oxidative injury to feline red blood cells has been reported.
Patient infections related to the use of propofol have been reported. This is thought to be due to microbial contamination of propofol and has resulted in life-threatening sepsis and postoperative infections of clean wounds in both human and veterinary patients.18,97 Propofol was found to be an excellent medium for rapid bacterial growth.209 Current recommendations are to discard unused propofol 6 hours after a vial or ampule is opened.73
Clinical Use
Initial clinical studies in cats reported the induction dose as 6.8 mg/kg in unpremedicated cats and 7.2 mg/kg in premedicated cats.24 In some studies, premedication reduced the dose by up to 60%,148,201 while in other studies premedication did not affect the induction dose.24,234 In a large clinical trial, the dose in unpremedicated cats was reported as 8.03 mg/kg and in premedicated cats as 5.97 mg/kg.148 Apnea after induction has been reported in all animal studies and is minimized by slow administration. The time from administration of the last dose to walking was reported as 27 to 38 minutes, depending on premedication and top-up doses. Recovery was rapid and usually excitement free.
Side effects have been reported in all animal studies. In cats an incidence of 14% was reported, with retching, sneezing, and pawing at the eyes and mouth the most prominent effects.24 The incidence was decreased by premedication with acepromazine.24
Pharmacodynamic Effects
There are no in-depth studies reporting the cardiopulmonary effects in cats, although in an early clinical study no changes were reported in heart rate or respiratory rate.234 In other species propofol is depressant, causing a fall in arterial blood pressure and cardiac output. It is not recommended for use in human patients with cardiac disease or hypovolemia. The arrhythmogenic threshold in cats induced and maintained with propofol increased compared with that of cats induced with either thiopental or propofol and maintained with halothane.72
Like thiopental and etomidate, propofol induces a dose-related depression of cerebral metabolic oxygen consumption rate and, presumably because of preserved cerebral autoregulation, reduces cerebral blood flow.215 As a result of the reduced cerebral blood flow and accompanying fall in cerebral blood volume, cerebrospinal fluid pressure is reduced. With propofol cerebral perfusion pressure may decrease as a result of a fall in arterial blood pressure, and care is required to minimize the fall so that cerebral perfusion is not compromised.
Propofol, like ketamine, demonstrated bronchodilating properties in the isolated guinea pig trachea167 and is considered a suitable induction agent for cats with asthma.
Propofol lowers gastroesophageal sphincter pressure and gastric pressure in cats, although this effect was less than reported with Saffan or thiopental.89
Propofol was reported to provide good conditions for semen collection by way of ejaculation in cats, in that ejaculation did not induce stress, onset of anesthesia was rapid, and recovery was smooth.35
Care should be taken if propofol is administered to cats over consecutive days because oxidative injury to cat red blood cells has been reported.8 Administration of propofol daily for 6 days resulted in an increase in Heinz bodies on day 3. Five of the six cats developed generalized malaise, anorexia, and diarrhea, and two cats developed facial edema.8 If anesthesia is restricted to a single induction dose, behavioral effects were not reported after daily administration for 4 weeks, although increases in methemoglobinemia and Heinz bodies were observed.73
Pharmacokinetic Effects
Initial studies reported a lower utilization ratio (0.19 mg/kg/min) in cats compared with other species.71 The utilization ratio was reported as the amount of drug administered divided by the duration of anesthesia. Differences in utilization ratio were likely related to differences in the rate of biotransformation and conjugation, insofar as the cat has a deficiency in its ability to conjugate phenols.71 In laboratory animals the initial distribution volume was large and redistribution to other parts of the body was extremely rapid. The total apparent volume of distribution was large, as was metabolic clearance from the body, with elimination half-lives in the range 16 to 55 minutes.2 In this study the slowest elimination was found in the cat.2 In most species the drug is reported to be noncumulative, making it an excellent drug for maintenance of anesthesia. In cats, when recovery to walking was compared among an induction dose only, an induction and maintenance dose for 30 minutes, and an induction and maintenance dose for 150 minutes, a significant increase in recovery time was reported for the latter dose.164 This provides further evidence that cats have reduced capacity to conjugate propofol, and thus recoveries will increase with increasing duration of propofol anesthesia.
Pulmonary extraction of propofol has been studied in cats and is substantial.132 This uptake is decrease by concomitant administration of halothane or fentanyl.
Etomidate
Etomidate is an imidazole derivative that is soluble in water but not stable, so it is formulated as a 0.2% solution in propylene glycol (35% by volume) with a pH of 6.9 and an osmolality of 4640 mOsm/L. It is more expensive than other induction agents and therefore is not used extensively in veterinary practice. However, in certain circumstances it does offer advantages in cats (Box 7-11).
BOX 7-11 Advantages and Disadvantages of Etomidate
Advantages
• It is a rapidly acting agent, with loss of consciousness occurring in 15 to 29 seconds. In situations in which a rapid sequence induction technique is required, etomidate is a suitable agent.
• It has an ultrashort duration of action, depending on administered dose, with a relatively rapid recovery. It is also suitable for nonpainful procedures of short duration.
• The relatively short elimination half-life and rapid clearance of etomidate make it a suitable drug for administration in a single dose, in multiple doses, or as a constant-rate infusion. Its adrenocortical suppression, however, limits its use to a single dose.
• It is the recommended induction agent when hemodynamic stability is important. It has been recommended in veterinary patients with preexisting cardiovascular disease or cardiac rhythm disturbances. It is a useful induction agent in cats with severe cardiac disease.
• It induces minimal respiratory depression and thus is a suitable agent when ventilatory stability is important.
• It decreases intracranial pressure in patients with raised intracranial pressure and is a good induction agent when there is concomitant cardiovascular disease or hypovolemia from trauma.
• Etomidate is an effective anticonvulsant; however, because it may activate a seizure focus, caution is advised in cats with epilepsy.
• It decreases intraocular pressure and is a good induction agent in cats with descemetoceles or corneal lacerations associated with other systemic trauma.
Disadvantages
• It is the most expensive of the injectable anesthetic agents.
• In the commercially available solution, the diluent is 35% propylene glycol, which can cause hemolysis, pain on injection, and thrombophlebitis.
• Induction and recovery may not be smooth and may include myoclonus and excitement.
• Adrenocortical suppression follows both induction and maintenance doses. Although its use as an induction agent is considered safe, it should not be administered as an infusion for maintenance of anesthesia.
• Some authors suggest that, in animals dependent on corticosteroids, a physiologic dose of dexamethasone or any other short-acting glucocorticoid should be administered if anesthesia is induced with etomidate.
Clinical Use
After 3 mg/kg intravenously, in which half was given rapidly and the remainder over 1 minute, induction of anesthesia was rapid and smooth. Recovery was also rapid, although a brief period of myoclonia was observed in all cats early in recovery.239
Pharmacodynamic Effects
There are few reports concerning the pharmacologic effects of etomidate in cats. In all species etomidate has minimal effects on the cardiovascular and respiratory systems. In dogs heart rate, blood pressure, and cardiac output were unchanged after administration of 1.5 or 3 mg/kg of etomidate.155 Similarly, 1 mg/kg of etomidate induced minimal changes in hypovolemic dogs.165 It also appears that etomidate has minimal effects on the cardiovascular system in cats.207
Etomidate induces a dose-related depression of cerebral metabolic oxygen consumption rate and, presumably because of preserved cerebral autoregulation, reduces cerebral blood flow.144 As a result of the reduced cerebral blood flow and accompanying fall in cerebral blood volume, cerebrospinal fluid pressure is reduced. With etomidate cerebral perfusion pressure is not compromised because intracranial pressure decreases more than mean arterial pressure.
Similar to other induction agents, etomidate decreases gastroesophageal sphincter pressure and barrier pressure and thus may predispose cats to regurgitation under anesthesia.89
Etomidate causes hemolysis, even after a single induction dose.237 The mechanism is thought to be the rapid increase in osmolality caused by the propylene glycol, causing red blood cell rupture. Caution should be exercised in patients with renal insufficiency because of the increased pigment load brought about by hemolysis.159
Induction of anesthesia with etomidate (2 mg/kg intravenously) in cats caused suppression of adrenocortical function during 2 hours of halothane anesthesia and for 1 hour in recovery. An additional 2 hours were required for cortisol to return to baseline.147 The impact of adrenocortical suppression after etomidate administration on long-term morbidity and mortality has not been determined. Some authors suggest that, in animals dependent on corticosteroids, a physiologic dose of dexamethasone or any other short-acting glucocorticoid should be administered if anesthesia is induced with etomidate.129 Adrenocortical suppression, however, precludes the administration of etomidate as an infusion for maintenance of anesthesia.
Pharmacokinetic Effects
The pharmacokinetics of etomidate in cats has been reported.239 The drug has a rapid distribution (half-life 0.05 hour); a large volume of distribution at steady state (4.88 L/kg); and rapid clearance (2.47 L/kg/h), which accounts for its short duration of action and rapid recovery.239
Steroid Anesthetics
The progesterone derivative, alphaxalone, is a neuroactive steroid anesthetic agent (Box 7-12). It was first introduced into veterinary anesthesia in 1971 as a component of the drug Saffan, an anesthetic agent in cats. Alphaxalone was insoluble in water and was mixed with alfadolone acetate to increase its solubility. Alfadolone also had anesthetic properties, with about half the potency of alphaxalone. Saffan is formulated such that the mixture contains 9 mg/mL of alphaxalone and 3 mg/mL of alfadolone, with the solubilizing agent as 20% polyethoxylated castor oil (Cremophor EL). The recommended dose is either expressed as mL of formulated solution/kg or mg of combined steroid/kg. Despite widespread use in other countries, Saffan or its medical counterpart, Althesin, were never available in the United States. Cremophor EL causes histamine release in animals by stimulating mast cell degranulation, and this was responsible for unacceptable adverse events.40,49,62 In humans the incidence of anaphylactoid reactions was high, and Althesin was removed from the market.
Recently, an Australian company has reformulated alphaxalone in hydroxypropyl beta cyclodextrin (Alfaxan), and it is commercially available for use in dogs and cats in Australia, New Zealand, South Africa, and the United Kingdom.150 Two recent publications have reported the pharmacokinetics and cardiorespiratory and anesthetic effects of this drug in cats.150,241
Clinical Use
The initial dose of Saffan is calculated on a body weight basis; however, the drug is titrated to effect. The intravenous induction dose is 0.75 mL/kg (9 mg/kg). Generally, one half of the calculated dose is administered over 20 to 30 seconds, and the patient is observed for drug effects. If more drug is required, a quarter of the calculated dose is administered over 20 to 30 seconds, and this is repeated until the desired anesthetic depth is accomplished. Intravenous injection produces unconsciousness in 10 to 25 seconds, and the depth and duration of surgical anesthesia is dose dependent. Return of righting reflex took 7, 17, 44, 75, and 136 minutes after doses of 1.2, 2.4, 4.8, 9.6, and 19.2 mg/kg, respectively.37 After the recommended intravenous dose of 9 mg/kg, relaxation occurs in 9 seconds and surgical anesthesia in about 25 seconds. Anesthesia is usually maintained for about 10 minutes, and recovery is rapid.116
The calculated dose for the newly released, reformulated alphaxalone (Alfaxan) is 5 mg/kg, with premedicants decreasing the dose to 2 to 3 mg/kg.114 After administration of 5 and 15 mg/kg doses, induction of anesthesia was characterized as quiet, uneventful, and relaxed. Time to lateral recumbency was inversely proportional to the dose of alphaxalone administered. The average time to lateral recumbency was approximately 15 to 30 seconds. Recovery scores for the 5 and 15 mg/kg doses of alphaxalone were excellent and not different from each other. Doses 10 times the induction dose were invariably fatal.150
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree