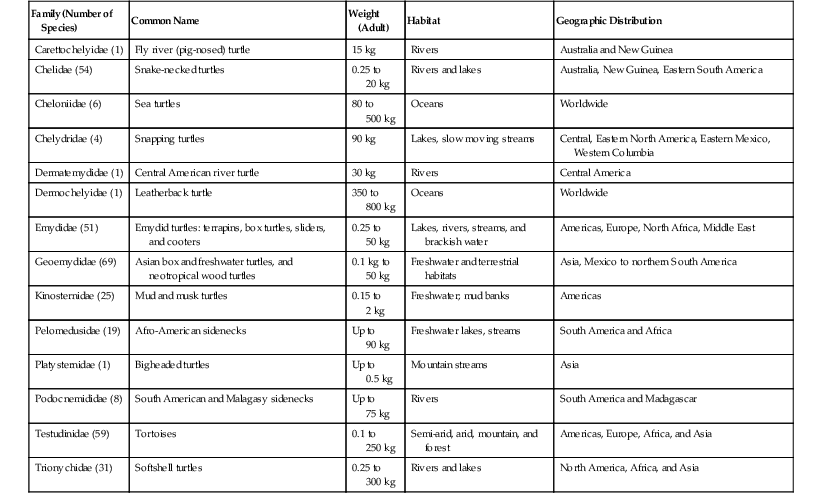
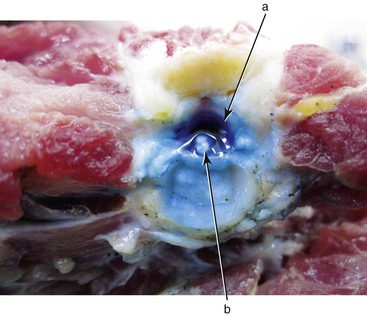
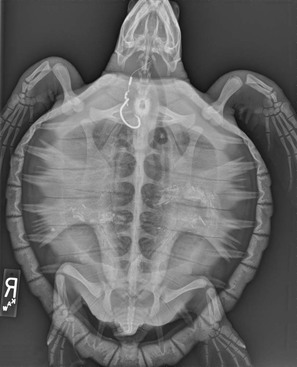
Joseph P. Flanagan Currently, 322 living species of tortoises and freshwater and marine turtles are in existence (Table 4-1). They are found in diverse habitats on all continents except Antarctica and in all oceans except the Arctic. The largest concentrations of turtle species occur in North America, more than in any other temperate region of the world. Ninety-eight species of chelonians are endangered or critically endangered because of habitat degradation, human encroachment, and harvesting for food, medicines, and the pet trade. Of the chelonians with known threat status in 2010, 53% of the species are considered threatened, making chelonians the most highly endangered of any of the major vertebrate groups.8 Chelonians range in size from the 100-gram (g) speckled tortoise (Homopus signatus) to the leatherback turtles (Dermochelys coriacae) weighing more than 800 kilograms (kg). Turtles and tortoises may be instantly recognized by the presence of a shell comprising the carapace (dorsal) and the plastron (ventral), which are joined laterally at the bridge. In most species, keratinized epithelium overlies a rigid bony structure that provides protection. The extent of shell coverage, flexibility, degree of mineralization, fenestration, hinging, and epithelial coverage vary. Most species have keratinized epithelium covering a thin dermis layer consisting of collagen fibers, melanophores, vessels, and nerves, beneath which is dermal bone.4 Most terrestrial species have firm, dense shells such as those of gopher tortoises (Gopherus polyphemus). Loss of bone in the central portions of the carapace and plastron of the pancake tortoise (Malacochersus tornieri) is normal and allows the species to wedge itself into rock crevices, where it may inflate itself to escape predation. Shells may be flexible at the bridges, which allows the hinged plastron of some species (Terrepene spp., Cuora spp., Kinosternon spp.) to seal tight against the carapace, whereas tortoises in the genus Kinyxs have hinged carapaces for protection. Certain aquatic species such as snapping turtles and certain species of the genus Kinosternon have reduced plastrons providing minimal rigid protection of the limbs. Vertebrae are incorporated into the carapace from the first thoracic vertebra caudally to the coccygeal vertebra. Sea turtles are unable to retract their heads and necks fully under their shells. Retraction of the head and neck may be in a horizontal plane as in the Pleurodiran species (side-necked and snake-necked turtles), or in a vertical plane by direct caudal retraction as is done in Cryptodiran species (typical of all land tortoises and most freshwater species of the northern hemisphere). Chelonian limbs are highly variable between species groups. Sea turtles and the freshwater pig-nosed turtle (Carettochelys) have flattened limbs that are highly adapted to an aquatic existence but that serve very poorly for walking on land. Most freshwater turtle species have varying degrees of interdigital webbing to facilitate swimming but retain the ability to walk on land. Some aquatic species such as snapping turtles are not good swimmers but “walk” on the bottom substrate during normal locomotion. Fully terrestrial species (Testudinadae) may have flattened forelimbs for burrowing and tend to have elephantine hindlimbs adapted for walking on land. The renal-portal system directs blood from the caudal region of the body through the kidneys or may divert it into the central circulation. Blood is shunted through the kidneys in times of water deprivation; however, pharmacokinetic studies have shown no significant difference in drug metabolism if injections are given in the caudal region versus the cranial limbs.3 All chelonians are considered ectothermic, although leatherback sea turtles are somewhat homeothermic. Preferred optimal temperatures differ according to species and should be considered in housing chelonians. Temperatures in the natural environment change according to diurnal and seasonal cycles. Captive animals should not be subjected to constant temperatures but should be provided with a diurnal fluctuation and a thermal gradient. Most turtles and tortoises depend on behavioral adaptations such as basking in the sun, seeking shade, or entering water or burrows to elevate, maintain, or decrease body temperatures. Immune function is enhanced when animals may achieve their preferred optimal temperature. For highly aquatic species, maintaining appropriate water temperature ranges is critical for behavioral reasons and for physiologic functions such as digestion. Chelonians may demonstrate episodic breathing and may shunt blood to or away from the lungs via vascular and intracardiac shunting mechanisms. Additionally, species adept at breath-holding demonstrate adaptations such as tolerance of hypoxia, large lung volume, rapid and extensive air exchange during ventilation, and physiologic buffering by bone, blood, and pericardial fluid of lactic acid and hydrogen ions built up during anaerobic metabolism. In aquatic species, gas exchange may also occur through the integument, pharynx, or cloacal tissues. Soft-shelled turtles may obtain up to 70% of their oxygen during submergence through their leathery shell.9 The kidneys of turtles have fewer nephrons than those of mammals and no loop of Henle, which creates an inability to concentrate urine. Nitrogenous waste is excreted as ammonia, urea, or uric acid. Proportions vary according to the biology of the species. Marine turtles and highly aquatic freshwater turtles may excrete up to 25% of their nitrogenous waste as ammonia. Other aquatic turtles may excrete primarily urea. Tortoises excrete most nitrogenous waste as relatively insoluble uric acid to prevent water loss. Water may be held in the urinary bladder and resorbed into general circulation. The ability of tortoises to store water in this manner enhances survival in xeric habitats and during times of drought. When handling tortoises in the wild, care must be taken to prevent the animals from urinating because this could result in significant losses of fluids that the animal might not be able to replace. The diverse species of chelonians demonstrate great diversity in diet and feeding habits. They may be omnivores, eating a broad spectrum of foods to constitute a complete diet, or they may be specialist feeders and have a strict, narrow spectrum of food items that they will accept. They may be herbivores, omnivores, or carnivores. Many have feeding strategies that change during different life stages. Wild-caught animals may be very difficult to acclimate to novel food items, and some may never willingly transition to captive foods. It is important to be familiar with the natural history of certain “specialized feeders” to provide familiar and acceptable food items while transferring animals to a balanced captive diet. Tortoises are herbivores that tend to graze on grasses and annual forbs but have very little natural exposure to fruits. Freshwater turtles are primarily omnivorous, consuming fish, crustaceans, snails, aquatic grasses, fallen fruits, and many other food items. Some are more carnivorous as juveniles but become more herbivorous with age. Complete rations have been formulated specifically for different groups of animals, and some may be used successfully for all life stages. Like other aquatic turtles, sea turtles may be omnivorous, carnivorous, or herbivorous. Green sea turtles feed on sea grasses and algae. Loggerhead and Ridley sea turtles prefer a diet of molluscs and crustaceans, whereas hawksbills specialize on sponges and leatherback sea turtles consume primarily jellyfish. Captive sea turtles will eat fish, crustaceans, and molluscs and may be acclimated to balanced diets in pellet or gelatin form. Obesity is not uncommon in captive turtles. One should remember that in nature, chelonians may spend a considerable amount of time foraging as a daily activity and such activity may provide benefits for well-being beyond simple nutrition. In general, hatchling chelonians of all species should be fed daily. Frequency of feeding may be reduced with age, but most species should be fed at least twice per week as adults. Most chelonia are relatively easily restrained. A potential risk exists for handlers from bites, scratches from claws, or cuts from projections of scales or points on the shell. Green sea turtles may flap their front flippers forcefully enough to fracture their humerus and may easily injure handlers while struggling. It is generally most safe to lift larger animals, placing one hand on the anterior carapace over the neck and the other hand on the caudal carapace over the tail. This minimizes the potential for bites or scratches. For species that may be lifted easily, care must be taken not to flip them upside down rapidly. This may cause immediate changes in hemodynamics that may cause distress to the animal, may impair ventilation because of compression of the lungs under the weight of coelomic viscera, or result in intestinal or uterine torsion. Aggressive animals may require diversion, immobilization, or blocking of the head to safely examine them. Materials such as rolls of tape or padded sticks or polyvinyl chloride pipes serve well as bite blocks placed in the mouth and secured with tape around the head. For small species, pushing the head under the carapace in a natural position and maintaining it there manually or with padding covered and taped to the shell allows nonpainful procedures on other portions of the body to proceed in safety. Many tortoises will not struggle if their eyes are covered or the heads are in the confines of their shells. Conversely, when chelonians are regressed completely into their shells, extricating limbs or the head without harming the animal is challenging. Some animals will extend their limbs when securely balanced on a pedestal, allowing for visual examination of the appendages and potentially permitting restraint of a limb or the head and neck. Using a small metal spatula, usually, even the tightest hinged plastron of a box turtle or the armored front leg of a tortoise may be breeched. Gentle, steady traction on the closed plastron or leg is usually rewarded by relaxation of the defense mechanisms. In extreme cases, chemical immobilization must be used to examine or treat the animal. General anesthesia may be induced and maintained with parenteral agents given through intravenous or intramuscular routes (Table 4-2). Gas anesthetics may be used to induce and maintain anesthesia, but induction under voluntary respiration may be prolonged when animals hold their breath. Direct intubation of the glottis may be accomplished in the awake chelonian through use of an oral speculum. The glottis is located at the base of the tongue and is closed unless the animal is taking a breath. An appropriate sized endotracheal tube may be gently forced into the glottis and the animal induced using positive pressure ventilation until an acceptable level of anesthesia is achieved. The trachea bifurcates into paired bronchi in the cranial one third of the cervical area and has complete cartilagenous rings. Care should be taken to prevent unilateral bronchial intubation. Anesthetic agents are metabolized more slowly under low body temperatures, so special attention should be given to maintaining the anesthetized chelonian at or near its preferred optimal temperature range. The turtle’s ability to withstand prolonged anoxia may cause reduced ventilation, resulting in reduced exchange of anesthetic gases. Whenever possible, animals under anesthesia should be intubated and provided with assisted ventilation. Recovery after gas anesthesia may be prolonged because ventilation is not induced by hypercapnea and decreases during hyperoxia. The use of room air rather than oxygen will speed anesthetic recovery.7 The use of local anesthetic (lidocaine 2% or bupivacaine 0.5%) may be used as adjunct to general anesthesia. Epithecal anesthesia (2% lidocaine at 1 mL/20 kg) may be used alone or in conjunction with general anesthesia for surgeries of the cloaca and tail (Figures 4-1 and 4-2).6 TABLE 4-2 Sedative, Anesthetic, Analgesic, and Restraint Agents Used in Chelonians IM, Intramuscularly; IV, intravenously, PO, per os. Surgical procedures should be performed under appropriate anesthesia and aseptic conditions. Intracoelomic surgery such as ovariectomy, salpingotomy for removing retained eggs, or enterotomy for removing a foreign body may be achieved by accessing the coelom through the prefemoral fossa. Incision size may be kept to a minimum by using rigid endoscopy to retract the ovarian tissue, oviduct, or intestine and bring it to the skin incision. Intracoelomic access may be limited via this route, however, and surgical objectives may require cutting through the plastron. In brief, the shell is cut to make a beveled edge by using a drill or a saw while attempting to keep a portion of the cut shell attached by preserving the periosteum. Care is taken to identify the coelomic blood vessels before incising them. With the shell out of the way, surgery may proceed as for any routine abdominal procedure. After the primary surgical objectives are met, closure of the coelom is accomplished by replacing the shell with the use of the beveled edge to help position and stabilize the flap during closure. The flap is affixed to the plastron using materials such as bone cement, acrylic, screws, and cerclage wire, in combination or alone. As with any orthopedic manipulation, complete healing of the shell may take months. Plastrotomy is not advised in species having dynamic movement of the plastron during normal respiration or diving (e.g., sea turtles), as the surgery site will be in constant motion, impeding or preventing the healing process. Fish hooks are commonly ingested by sea turtles and frequently lodge in the lower esophagus near the base of the heart. These may generally be accessed through a ventral cervical incision, retracting the esophagus cranially to access the site where the hook has lodged (Figure 4-3). Orthopedic procedures are similar to those for other animals. The density of long bones is generally greater than for mammals, and external splints may be challenging to maintain. Limbs may be taped inside shell openings for crude but effective means of immobilizing fractures of extremities. Shell fracture is a common presentation of animals hit by automobiles or speedboats. The fracture site is frequently grossly contaminated with dirt or water, with contamination of the lungs or coelomic cavity being a potential risk. The wound should be cleaned and the fragments stabilized by using support bars or cerclage wire, which will allow treatment of the fracture site as an open wound. Large defects with loss of shell fragments may be treated with vacuum-assisted closure. Healing time may take up to a year for bony bridging and reepithelialization. It may be daunting to try to perform a physical examination on a chelonian that is completely and firmly withdrawn into its shell, but the foundation of diagnosis lies with a good physical examination in combination with the history and signalment of the patient. Ideally, the patient should be examined in its primary enclosure, where it may be observed swimming or ambulating. In aquatic species, buoyancy, limb use, respiratory pattern, and environmental awareness have to be assessed. Once “in hand,” the animal should be weighed. Healthy chelonians often seem slightly heavier than expected when picked up. If an animal feels light, it may be malnourished or dehydrated. It is possible to overpower most smaller individuals, but patience and gentle, firm handling will often yield excellent results. Visual examination of the head, neck, limbs, and tail should help assess symmetry, skin condition, presence or absence of scars, ectoparasites, excess skin, overgrown nails, and presence and density of epibionts (algae and other commensal organisms) on the carapace. Presence of an abundance of epibionts may indicate chronicity of a problem. Thick algae on the carapace of a basking species may indicate that it has not hauled out to thermoregulate, and high numbers of barnacles on sea turtles may indicate prolonged lethargy or reduced activity, as healthy turtles tend to have cleaner shells. Evaluation of the gastrointestinal (GI) system starts with the oral cavity (often easily done with more aggressive species!) for abnormal mucosa or foreign bodies (fish hooks are common in some species). The cloaca is examined for swelling, foreign bodies, or trauma. Evaluation of the respiratory system is done through examination of the nares, choana, and glottis. This is followed by listening to respiration and auscultating the lung over the dorsum of the carapace. Percussion of both right and left lungs should sound the same. Neurologic evaluation includes observation of posture, carriage of head, symmetry of muscle mass, tone, and strength. Reflexes may be evaluated using tactile stimuli such as a toe pinch. Eyes should be clear and symmetrical, with no discharge or swelling of adnexa. The tympanum, located just caudal and ventral to the eye should be flat and bilaterally symmetrical. Abdominal palpation performed in the prefemoral fossae may reveal the presence of coelomic masses, ova, bladder stones, and ascites. Blood samples should be drawn into tubes or syringes containing the anticoagulants sodium or lithium heparin. Ethylenediaminetetraacetic acid (EDTA) should not be used because it causes hemolysis, which precludes obtaining accurate hematocrits and electrolyte determination. In addition, blood smears do not stain optimally with EDTA compared with heparin. Several sites are easily accessible for blood collection. Venipuncture of the jugular is the most reliable site for uncontaminated peripheral blood samples. The jugular vein is easily visualized in most species when the head and neck are held in extension. Extending the head and neck may be challenging in strong or aggressive species and may require tranquilization to prevent injury. Sample contamination with lymph is common to most venipuncture sites other than the jugular vein. The dorsal coccygeal vein requires the least overall restraint and is located just dorsal to the dorsal aspect of the vertebral body on the tail. The needle is introduced into the dorsal skin at a 45- to 90-degree angle to the vertebra. Vascular access to the axillary branch of the brachial vein in the forelimb may be achieved by extending the animal’s front leg and inserting a needle through the skin distal to the carpus and between the carpal flexor tendons aiming toward the posterior side of the carpal joint. The subcarapacal sinus is located just under the carapace immediately caudal to the last cervical and cranial to the first thoracic vertebrae. The venipuncture site may be accessed by pushing the head down and into the shell then palpating the bony prominence of the vertebra where it meets the carapace. The needle is inserted on the midline just caudal to the juncture of the skin with the carapace, aiming toward the carapace just cranial to the vertebral prominence. In sea turtles, the dorsal cervical sinus (supravertebral), located one third the distance from the carapace to the base of the skull, affords a reliable site for uncontaminated blood collection with minimal restraint. The head is directed forward and down, and an appropriate length needle (up to The occipital sinus may also be used for blood collection, but this requires that the head be restrained firmly in an extended position and tilted down at a 45-degree angle to the spine. A needle is introduced just caudal to the occipitus, perpendicular to the spine. Lymphatic contamination sometimes is encountered at this site. Complete blood cell counts generally are performed using manual cell-counting techniques, although automated methods have been used for red blood cell (RBC) counts. Hemoglobin determination is accomplished by serometer, hemoglobinometer, or automated methods. Microhematocrit centrifugation is the standard for packed cell volume (PCV) determination. RBCs are nucleated and range in number from 0.154 to 0.980 × 106/µL, depending on species and time of year. RBCs have long life spans, with the mean in box turtles being 600 to 800 days. Peak reticulocyte response to blood loss takes up to 5 weeks to achieve. Because turtles have a total blood volume of 5% to 8% of total body weight and the standard procedure is to limit blood collection to 10% of the total, restricting sample size to 0.5% to 0.8% of the body weight (0.5 to 0.8 mL for a 100-g animal) is appropriate. Drawing blood frequently over a short period or in excess of recommended volumes may cause iatrogenic anemia, which corrects slowly. White blood cell (WBC) differentiation is best performed on smears that have been made immediately after collection. Heparin is the preferred anticoagulant because of less distortion of WBC morphology. Blood smears made in the field do not stain well if staining is delayed more than a few days, even when they are fixed soon after being made. For best results, smears should be stained within a few hours of being made. Total WBC counts may be determined by the direct method (Natt-Herricks) or indirectly (phloxin B solution or estimation from smear). The most reliable results are obtained by consistent processing and analysis. All methods require some level of technical skill to achieve accuracy, and methods may not be comparable directly one with another within or between laboratories. It is ideal to use one laboratory and one or two technicians with similar training and skills, as significant variation may occur between laboratories because of the interpretations of the technicians handling the samples. The chelonian leukocyte response is less predictable than in mammals or birds. Normal (reference) values are hard to establish because of variations by species, season, nutritional status, type of stain, venipuncture site, handling of sample, age, sex, and anticoagulant used. Twofold changes in a parameter constitute a significant change. The best use of hematologic values is to monitor a patient’s response to therapy. Blood values change seasonally, especially with species that undergo brumation (hibernation). For example, total RBC counts are highest before brumation and lowest immediately thereafter. Other parameters change as well and should be considered relative to the environmental conditions and sample handling and processing. Heterophil counts increase during summer and decrease during brumation. Increased counts may indicate inflammation or bacterial disease. “Toxic” heterophils display cytoplasmic basophilia, abnormal granulation, and a lobed nucleus and are present in cases of inflammation. Eosinophilia may be seen in parasitic disease. Basophilia may be present in parasitic infection as well as in viral disease processes. Lymphocyte counts are low to absent in the winter and are low in cases of malnutrition and in diseases secondary to stress and immunosuppression. Lymphocytosis is seen in wound healing, parasitic disease, and viral infections. Monocyte numbers increase in granulomatous inflammation. Reference ranges for selected chelonian species are available elsewhere.1 Biochemistries may be determined on plasma or serum. Whole blood should be cooled or centrifuged within 15 minutes of collection to prevent changes from RBC metabolism such as loss of potassium into the plasma. The anticoagulant EDTA causes changes in potassium and calcium directly and in other parameters through the effects of hemolysis. Serum biochemical parameters are similarly affected by the factors that affect hematology values. Blood samples yield a higher volume of plasma compared with serum, so biochemical assays are routinely run on plasma. Often, blood samples are obtained with a varying degree of lymph contamination. Values of glucose, calcium, phosphorous, sodium, urea, and enzymes in lymph are comparable with those in plasma. Lymph is lower in total protein and potassium compared with plasma. Assessment of renal function in chelonians is more difficult because of the physiologic differences between freshwater, saltwater, and terrestrial species. Blood urea nitrogen (BUN) and creatinine are generally poor indicators of renal disease. Values are generally low (<40 milligrams per deciliter [mg/dL]) in terrestrial and freshwater species and higher in marine species (~100 mg/dL) and terrestrial species during dry season when they are conserving water. A low value in marine species may be an indicator of prolonged anorexia. Plasma uric acid levels in chelonians are generally lower than 5 mg/dL. Elevations may be seen in cases of bacteremia, septicemia, nephrocalcinosis, and nephrotoxicity but may also represent gout or the recent ingestion of a high-protein diet. Sodium levels range from 120 to 150 milliequivalents per liter (mEq/L) in tortoises and freshwater turtles and from 150 to 170 mEq/L in sea turtles. Hyponatremia may result from GI or renal disease, oversupplementation of fluids low in sodium, disease of the salt gland, or maintenance of saltwater species in fresh water. Hypernatremia may occur in dehydration or excessive dietary intake. Potassium levels normally range from 2 to 6 mEq/L. They are elevated because of hemolysis and reduced renal secretion and are low because of reduced intake or excess GI loss. Normal blood pH ranges from 7.5 to 7.7 at temperatures of 23° C to 25° C. Increasing temperature will reduce blood pH, and prolonged anesthesia will increase it. Blood calcium levels range from 8 to 11 mg/dL. Levels may increase two to four times because of follicular development. Levels less than 8 may be caused by anorexia, dietary deficiencies of calcium or vitamin D3, hypoalbuminemia, alkalosis, or hypoparathyroidism. Normal phosphorous levels range from 1 to 5 mg/dL. Low levels are caused by starvation or nutritional deficiency. Hyperphosphatemia results from excessive dietary phosphorous, hypervitaminosis D, renal disease, or severe tissue trauma or may be falsely elevated because of leakage from RBCs when not separated quickly enough from serum or plasma. Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) activities are high in chelonian liver tissue but are not specific for this organ. AST is found in many tissues. Levels above 250 international units per liter (IU/L) suggest liver or muscle damage, septicemia, or toxicity. LDH levels above 1000 IU/L may be associated with hemolysis or with damage to the liver, heart, and skeletal muscle. Elevation of LDH and AST in the absence of elevation of creatinine kinase (CK) is indicative of liver disease. Plasma protein levels range from 3 to 7 g/dL. Low levels are seen in chronic malnutrition, protein-losing enteropathies, and maldigestion and in chronic liver and kidney disease. Elevated levels are most commonly seen during folliculogenesis but may also be seen in dehydration or may be caused by hyperglobulinemia associated with infectious disease. Plasma glucose normally ranges from 60 to 100 mg/dL. Low levels may be caused by starvation, malnutrition, hepatobiliary disease and septicemia. Elevated levels are most likely iatrogenic and caused by excess glucose administration or the administration of glucocorticoids. CK is a muscle-specific enzyme. Elevations occur with muscle injury from trauma, struggling, systemic infection, or administration of intramuscular injection of fluids or irritating drugs such as enrofloxacin. Reference ranges for selected chelonian species are available elsewhere.1
Chelonians (Turtles, Tortoises)
Biology
Unique Anatomy
Special Physiology
Feeding
Restraint and Handling
Surgery and Anesthesia
Generic (Trade) Name
Dose and Route
Comment
Atipamezole (Antisedan)
5× dose of medetomidine, 10× dose of dexmedetomidine
α-adrenergic reversal for medetomidine and dexmedetomidine
Bupivacaine
1 to 2 mg/kg local infiltration
4 mg/kg maximum dose
Local anesthetic
Buprenorphine
0.075 mg/kg SC
Effect lasts 24 or more hours
Butorphanol (Torbugesic)
0.2 mg/kg IM
Tranquilizer
Dexmedetomidine (Dexdomitor)
0.03 to 0.075 mg/kg
Anesthesia
Use in combination with ketamine
Reverse with atipamezole
Diazepam (Valium)
0.2 to 1.0 mg/kg IM
Use with ketamine for relaxation
Isoflurane (IsoFlo)
3% to 5%
Use face mask, chamber, or endotracheal tube
Long induction time
Ketamine HCl (Multiple)
5 to 25 mg/kg IV or 20 to 60 mg/kg IM
Highly variable response to IM dosing
Use lower doses IV or in combination with α-adrenergic and higher dose IM or when given alone
Lidocaine (0.05-2%) (Multiple)
Up to 2 mg/kg total dose, 1 mL 2%/20 kg for epithecal
Local infiltration or epithecal
Medetomidine (Dormitor; Zaloprim)
0.1 to 0.15 mg/kg IV or IM
Use with ketamine, 5 mg/kg
Reverse with atipamezole
Meloxicam
0.1 to 0.5 mg/kg PO, IM, or 0.22 mg/kg IV
Analgesia
Study in red-eared sliders
Better absorption IM versus PO
Rapid elimination after IV administration
Morphine
1.5 to 6.5 mg/kg SC
Analgesia
Higher dose has more rapid onset (2 hours versus 4), both last over 8 hours
Propofol (Deprivan)
5 to 10 mg/kg IV
Restraint for 30 to 60 minutes
May cause respiratory depression requiring intubation and positive-pressure ventilation
Tiletamine/zolazapam (Telazol)
5 to 10 mg/kg IV or IM
Prolonged recovery, generally insufficient as sole anesthetic
Reverse with flumazenil 1 mg/20 mg zolezepam IM or IV
Tramadol
10 mg/kg PO
10 mg/kg SC
Analgesia
Lasts 9 to 96 hours
Analgesia
Lasts 12 to 48 hours
Xylazine (Rompun)
0.1 to 1.25 mg/kg IV or IM
Variable results, not recommended
Reverse with yohimbine 0.125 mg/kg IM
Clinical Procedures
Physical Examination
 inches in large animals) is introduced lateral to midline on either side. Raising the turtle’s body relative to the head enhances filling of the sinus. Ultrasonography may be used to locate the vein if difficulty is encountered.
inches in large animals) is introduced lateral to midline on either side. Raising the turtle’s body relative to the head enhances filling of the sinus. Ultrasonography may be used to locate the vein if difficulty is encountered.
Diagnostics
Hematology
Biochemistry
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Chelonians (Turtles, Tortoises)
Chapter 4