Web Chapter 61 Infective endocarditis (IE) is an uncommon, often deadly, and sometimes difficult to diagnose disease in veterinary medicine. IE is caused by invasion of a microbe into the endothelium of the valves of the heart, resulting in proliferative or erosive lesions and consequently valvular insufficiency. The incidence of IE in dogs referred to a veterinary teaching hospital is low (0.09% to 6.6%) (Calvert, 1982; MacDonald et al, 2004). In contrast, 75% of cardiac disease in dogs is caused by myxomatous valve degeneration. IE in cats is extremely rare. In small animals the mitral and aortic valves are affected almost exclusively with nearly equal distribution. Patients often succumb to congestive heart failure (CHF), sudden death, or thromboembolic disease. Immune-mediated diseases, including polyarthritis and glomerulonephritis, also are common secondary sequelae. Identification of the offending organism is paramount for effective treatment with long-term antibiotic administration. Blood culture is especially helpful to identify the offending organism and determine the spectrum of antibiotic sensitivity. However, the incidence of culture-negative IE in dogs is high at 60% to 70% (MacDonald et al, 2004; Sisson and Thomas, 1984). This chapter considers IE in the dog and discusses the emergence of Bartonella as a cause of IE in dogs with blood cultures negative for traditional bacteria. Recent advances in diagnosis, treatment, and prognosis of IE in dogs also are reviewed. Bacteremia of any cause may predispose a patient to develop IE. Common causes of bacteremia are discospondylitis, prostatitis, pneumonia, urinary tract infection, pyoderma, periodontal disease, and long-term indwelling central venous catheters. Subaortic stenosis is the only structural heart disease known to predispose dogs significantly to development of aortic valve IE (Sisson and Thomas, 1984). Dogs with myxomatous valve degeneration rarely develop IE despite dental prophylaxis procedures or other causes of bacteremia and therefore are not likely to have a substantially increased risk of development of IE. In a retrospective study of 76 dogs diagnosed with IE compared with 80 control dogs without IE, no association was found between oral infection or recent dental procedure and the development of IE (Peddle et al, 2007). The role of immunosuppressive therapy (e.g., corticosteroids) as a predisposing factor for development of IE is controversial. In a study of IE in dogs only 1 of 18 dogs (5%) had been administered immunosuppressive therapy recently. However, an earlier study found that 17 of 45 dogs with IE (38%) received corticosteroids at some time during the course of disease (Calvert, 1982). Patients with IE tend to develop high titers of antibodies against causative microorganisms, and there is continuous formation of circulating immune complexes consisting of immunoglobulin M (IgM) and IgG as well as C3 (complement). Factors such as rheumatoid factor may impair the ability of complement to solubilize immune complexes and may lead to formation of large immune complexes. Extracardiac disease manifestations are caused by immune complex deposition and further complement activation and tissue destruction in the glomerular basement membrane, joint capsule, or dermis. Levels of circulating immune complexes are greatly reduced shortly after antibiotic therapy in humans with IE. Immune-mediated disease is seen commonly in dogs with IE; polyarthritis and glomerulonephritis were observed in 6 of 8 dogs (75%) and 4 of 11 dogs (36%), respectively, in a study by MacDonald and colleagues (2004). Middle-aged or older male dogs of medium or large-breeds are most commonly affected with IE. A majority (92%) of affected dogs weigh more than 15 kg (Sykes et al, 2006a). German shepherds were found to be predisposed to develop IE in a postmortem study. Other breeds that appear to be overrepresented are the boxer, golden retriever, and Labrador retriever. The most frequent presenting complaint of dogs with IE in a study of 18 dogs was lameness (44% of dogs), followed by nonspecific signs such as lethargy, anorexia, respiratory abnormalities, weakness, and collapse (MacDonald et al, 2004). Neurologic abnormalities (e.g., seizures, nystagmus, head tilt, obtundation), vomiting, and epistaxis are less common presenting complaints. There usually is not an identifiable recent precipitating factor such as a surgical or dental procedure, catheterization, or trauma, which is illustrated by the fact that fewer than half of dogs had an identifiable precipitating cause in a study of 18 dogs with IE (MacDonald et al, 2004). Given the acute nature of IE, the clinical signs tend to be present for a short time before diagnosis, with a median duration of illness before admission of 10 days in a large case series of 71 dogs with IE (Sykes et al, 2006a). A murmur is auscultated in a majority of dogs with IE (89% to 96%), although one study reported a lower incidence of murmurs of 59% (41 of 70 dogs) (MacDonald et al, 2004; Sisson and Thomas, 1984; Sykes et al, 2006a). Clinical findings of a diastolic murmur and bounding femoral pulses should trigger a high level of suspicion of aortic valve IE, and further diagnostic testing should be pursued immediately as outlined in the following paragraphs. Fever often is present (50% to 74% of cases); however, dogs with Bartonella-associated IE are more likely to be afebrile. Arrhythmias are present in 40% to 70% of dogs and include in order of incidence ventricular arrhythmias, supraventricular tachycardia, third-degree atrioventricular block, and atrial fibrillation. The highest reported frequency of arrhythmias was in dogs with aortic IE, with 62% of dogs having ventricular arrhythmias (Sisson and Thomas, 1984). Third-degree atrioventricular block may occur with periannular abscess formation secondary to aortic valve IE. CHF is present in almost half of patients and is diagnosed by identification of perihilar to caudodorsal pulmonary infiltrates and pulmonary venous distention. Acute CHF, often with widespread pulmonary edema, occurs in the absence of significant left atrial enlargement in a majority of cases of IE (75%) (MacDonald et al, 2004). In a large case series of 71 dogs diagnosed with IE, CHF was more likely to develop in dogs with aortic valve involvement (Sykes et al, 2006b). Thromboembolism (septic and aseptic) occurs in 70% to 80% of dogs with IE examined at pathology and in almost half of all clinical cases (MacDonald et al, 2004; Sykes et al, 2006a). Like people, dogs are more likely to experience thromboembolic disease with mitral valve IE (Sykes et al, 2006a). In people, the risk of thromboembolic disease is greatest with mitral valve IE, mobile vegetative lesions larger than 1 to 1.5 cm, or lesions that increase in size during antibiotic therapy (Macarie et al, 2004; Mügge et al, 1989). Infarction of the kidneys and spleen are most common in dogs, followed by infarction of the myocardium, brain, and peripheral arteries. Vascular encephalopathy occurs in approximately one third of people with IE but is uncommon in dogs. Recently a case series of four dogs with IE and vascular encephalopathy was described (Cook et al, 2005). Central nervous system thromboembolism most commonly occurs in the middle cerebral artery in both people and dogs and results in brain ischemia and possibly ischemic necrosis if persistent. Arthrocentesis, cytologic analysis of joint fluid, and culture of joint fluid are indicated procedures for dogs with lameness or joint effusion. In a study of 71 dogs with IE, 35% of dogs had joint fluid analyzed, and 84% of these dogs had suppurative effusion (Sykes et al, 2006a). Septic inflammation is less common than immune-mediated polyarthritis. There is a high incidence of negative blood culture results in dogs with IE ranging from 60% to 70% (MacDonald et al, 2004; Sisson and Thomas, 1984) The most common bacterial isolates are Staphylococcus spp. (S. aureus, S. intermedius, coagulase-positive, and coagulase-negative), Streptococcus spp. (S. canis, S. bovis, and β-hemolytic), and Escherichia coli (Web Table 61-1). Other isolates cultured at the author’s hospital and reported in other case series include Pseudomonas spp., Erysipelothrix rhusiopathiae, Enterobacter spp., Pasteurella spp., Corynebacterium spp., and Proteus spp. Rare bacterial isolates include a Bordetella avium–like organism, Erysipelothrix tonsillarum, and Actinomyces turicensis. WEB TABLE 61-1 Common Causative Agents, Typical Antimicrobial Sensitivity Profiles, and Treatment Recommendations for Dogs with Infective Endocarditis Polymerase chain reaction (PCR) testing of blood for bacterial nucleic acid has been used in people suspected to have IE. PCR testing of blood for bacterial 16s primers to identify bacteremia in dogs with suspected or confirmed IE was tested and compared with traditional blood culture in 18 dogs (Meurs et al, 2011). Blood samples for PCR testing, like samples for blood culture, should be obtained using sterile technique. Only 6 of 18 dogs had positive results on blood cultures, and similarly 7 of 18 dogs had positive PCR results for bacterial DNA. However, in only two of the dogs were bacteria identified by both blood culture and PCR testing of blood. When both PCR and blood culture were considered together, bacteremia was detected in 11 of 18 dogs (61%), and neither diagnostic modality proved to be superior. However, minimal inhibitory concentration (MIC) data are available only for blood samples that yield positive culture results and not for those yielding positive PCR findings, which limits the usefulness of blood PCR testing for antimicrobial selection. A PCR blood assay for Bartonella spp. is available commercially but sometimes may produce false-negative results as is seen with blood culture. This is likely due to a relapsing pattern of bacteremia and the localization of Bartonella organisms within endothelial cells or vegetative lesions without large numbers of circulating bacteria.
Infective Endocarditis*
Predisposing Factors
Pathophysiology
Immune-Mediated Disease
Clinical Presentation
Cardiopulmonary Abnormalities
Other Systemic Sequelae
Diagnosis
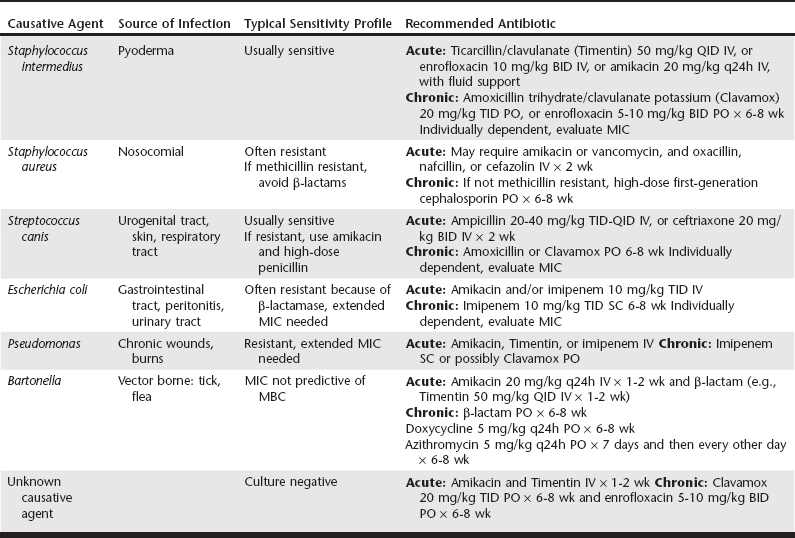
Bacterial Isolates
Polymerase Chain Reaction Assay for Bacteremia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Chapter 61: Infective Endocarditis
Only gold members can continue reading. Log In or Register to continue
