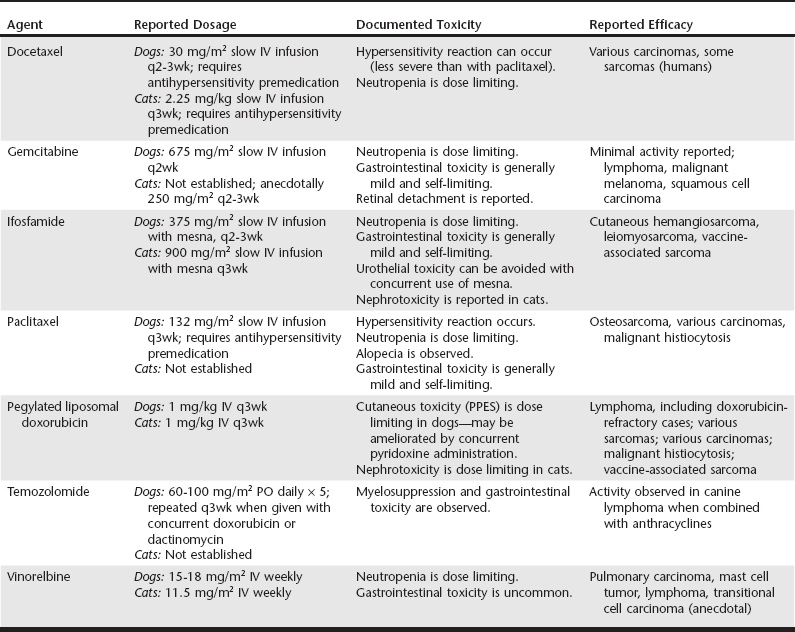
Web Chapter 25 Of the treatment modalities available in veterinary oncology, surgery remains the most commonly applied and the most likely to effect cure. However, local recurrence or distant metastasis results in the majority of cancer-related deaths. The use of antineoplastic chemotherapy has increased greatly in the last decade in both general and specialty veterinary practice. Well-designed clinical trials are being performed constantly to determine the appropriate dose, tolerability, and efficacy of novel antineoplastic drugs. General practitioners should be aware of this expanding knowledge base, whether they will be using these drugs in their practice or referring clientele to centers elsewhere to receive them. There is ever-increasing information regarding optimal doses, schedules, combinations, and routes of delivery of established chemotherapeutic agents in veterinary practice (see other chapters in this section). This chapter focuses on newer classical cytotoxic agents (rather than targeted agents, monoclonal antibodies, or antiangiogenic or immunomodulatory compounds) that have U.S. Food and Drug Administration approval for use in humans. A summary is provided in Web Table 25-1. For additional information, the reader is referred to other reviews (Chun et al, 2007; Moore and Kitchell, 2003). WEB TABLE 25-1 Newer Cytotoxic Agents in Veterinary Oncology IV, Intravenously; PO, orally; PPES, palmar-plantar erythrodysesthesia syndrome. Ifosfamide is an alkylating agent and its use has been evaluated in tumor-bearing dogs and cats (Rassnick et al, 2000, 2006a, 2006b). It must be given during a diuresis and with a thiol compound, sodium 2-mercaptoethane sulfonate (mesna), to prevent sterile hemorrhagic cystitis induced by acrolein, a metabolite of ifosfamide that is toxic to bladder urothelium. The recommended dose and delivery schedule in dogs is to give mesna (reconstituted in 0.9% NaCl to a concentration of 20 mg/ml) at 20% of the calculated ifosfamide dose. Mesna is given first as an intravenous bolus, and then the patient is diuresed with 0.9% NaCl at 18.3 ml/kg/hr for 30 minutes. Ifosfamide (375 mg/m2 reconstituted in 0.9% NaCl to a volume of 9.15 ml/kg of body weight) is then given IV over 30 minutes, followed by an additional 5 hours of saline diuresis (18.3 ml/kg/hr). Two additional mesna doses are given at 2 and 5 hours during the post-ifosfamide diuresis. Ifosfamide can be dosed at either 2- or 3-week intervals in dogs. Phase I and subsequent phase II studies suggested that ifosfamide can be given safely at dosages of 900 mg/m2 q3wk in cats using a similar administration protocol. In one study of 72 tumor-bearing dogs, an overall response rate of 6% was reported. Responses were noted in dogs with sarcomas, including cutaneous hemangiosarcoma and leiomyosarcoma (Rassnick et al, 2000). Only 1 of 40 dogs with lymphoma (LSA) responded. A protocol of alternating doxorubicin (DOX) and ifosfamide has been evaluated in dogs with hemangiosarcoma; when this protocol was compared retrospectively to treatment with single-agent DOX in dogs, there was no apparent improvement in outcome. Based on the published data, it is possible that the canine dose may be escalated further, which may improve antitumor activity. Ifosfamide appears to be an active drug for the treatment of feline vaccine-associated sarcoma; an overall response rate of 41% was reported in a recent phase II study (Rassnick et al, 2006b). Liposomes are closed vesicular structures that consist of one or more lipid bilayers. Several different types of liposome can be engineered with different physical properties that can dramatically alter drug pharmacokinetics or pharmacodynamics. Most liposomal formulations are designed to enhance antitumor efficacy, decrease normal tissue toxicity, or a combination of the two. Doxil, a liposomal formulation of DOX, has been used effectively in dogs and cats (Poirier et al, 2002; Vail et al, 1997). Unlike with free (unencapsulated) DOX, with the liposomal formulation myelosuppression and cardiotoxicity are not dose limiting. This makes Doxil a suitable drug for use in dogs with preexisting cardiac disease or in dogs that have received cumulative doses of free DOX that are approaching cardiotoxic levels. The dose-limiting toxicity in dogs is a cutaneous toxicity called palmar-plantar erythrodysesthesia syndrome (PPES), characterized by lesions ranging from mild erythema and alopecia to severe crusting and ulceration primarily in the axilla, inguinal region, and skin surrounding the footpads. The severity of PPES can be diminished by concurrent use of vitamin B6 (pyridoxine 25 to 50 mg q8h PO) throughout treatment. The dose-limiting toxicity of Doxil in cats is a delayed nephrotoxicity, although it occurs rarely at the recommended dosage. As with native DOX, adequate renal function is imperative in cats receiving the drug. The recommended dosage for Doxil in both dogs and cats is 1 mg/kg IV as a 10-minute bolus q3wk. Canine tumors reported to respond to Doxil include LSA, malignant histiocytosis, soft tissue sarcomas, mammary adenocarcinoma, anal sac adenocarcinoma, and squamous cell carcinoma. In addition, dogs with LSA resistant to native DOX have responded to Doxil, which implies some mechanism of abrogating drug resistance. Feline vaccine-associated sarcomas have been reported to respond to Doxil; however, native DOX appears as effective as the more expensive liposomal formulation (Poirier et al, 2002). There are also anecdotal reports of responses in cats with various carcinomas and cutaneous hemangiosarcoma. A recent study evaluated the efficacy of intraperitoneal Doxil for adjuvant treatment of canine splenic hemangiosarcoma (Sorenmo et al, 2007). There was no obvious improvement in outcome compared with the results of studies using conventional DOX–based protocols.
Anticancer Drugs
New Drugs
Ifosfamide (Ifex)
Pegylated Liposomal Doxorubicin (Doxil, Caelyx)
Chapter 25: New Drugs
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


