Web Chapter 23 Hyperinsulinism is best diagnosed by the interpretation of serum insulin and glucose concentrations obtained from the patient at the same time. If the clinician suspects hyperinsulinism at the time of initial examination of an animal with signs associated with hypoglycemia, serum samples for insulin levels can be obtained at that time. If attempts are made to document hyperinsulinism at a later date, samples should be obtained after fasting when the glucose is less than 50 mg/dl (Feldman and Nelson, 2004). Patients suspected of having hyperinsulinism must fast under supervision to allow intervention should signs of hypoglycemia occur. Samples for serum glucose determination should be collected in sodium fluoride. The insulin radioimmunoassay must be validated for the species of interest. Reference ranges vary among laboratories and species. When glucose is administered intravenously in patients with insulinoma, the tumor may be stimulated to release massive amounts of insulin, leading to severe hypoglycemia. This may result in a vicious cycle of the patient receiving larger volumes and more frequent dosing of intravenous dextrose even as clinical signs become more severe. In dogs with insulinoma, intravenous glucagon may be considered if the low serum glucose and associated clinical signs are not reversed with infusions of dextrose. Glucagon stimulates hepatic gluconeogenesis and glycogenolysis. According to package directions 1 mg of lyophilized glucagon USP should be reconstituted according to package directions and mixed with 1 L of 0.9% saline solution. This resulting 1 µg/ml solution is given at 5 to 10 ng/kg/min. The dosage is adjusted as needed to maintain the serum glucose at a concentration of 50 to 100 mg/dl. When the dog is able to eat and maintain its own blood sugar and/or other surgical or medical therapy is used to treat the insulinoma, the glucagon infusion may be tapered slowly over 1 to 2 days as the serum glucose and clinical signs are monitored (Fischer et al, 2000). Surgery is the treatment of choice for the initial long-term management of animals with insulinoma. Exploratory celiotomy is useful in confirming the diagnosis, staging the patient, and removing the neoplastic tissue. All identifiable pancreatic nodules should be removed, and metastatic lesions should be resected whenever possible. When possible, pancreatic masses should be removed by partial pancreatectomy. Survival time for dogs undergoing partial pancreatectomy is longer than that for those undergoing nodulectomy. Partial pancreatectomy can be performed by the suture-fracture technique, the dissection-ligation technique, or through the use of an electrothermal bipolar vessel-sealing device (LigaSure V). The bipolar vessel-sealing device (BVSD) denatures collagen and elastin within vessel walls and thus safely seals tissue and vessels while causing less tissue damage than is seen with the higher temperatures used in traditional cautery. Using the BVSD to perform partial pancreatectomy in dogs results in shorter surgical times and a decreased incidence of postoperative pancreatitis when compared with dogs undergoing the suture fractionation technique. The BVSD is likely more effective in sealing pancreatic ducts during partial pancreatectomy and thus minimized the leakage of pancreatic juices in to the remaining tissue that could cause local or generalized pancreatitis (Wouters et al, 2011). Whether metastatic lesions are visible, biopsy of the liver and mesenteric lymph nodes is recommended for staging. The serum glucose concentration should be stabilized before induction of anesthesia and surgery. Although it is not necessary for the serum glucose to be in the normal range, ideally the measured levels should be stable and the patient should have experienced one or more days without seizures before surgery. Frequent feedings, continuous intravenous infusion of dextrose solution (5% dextrose or higher), or both, are the best ways to accomplish this. If these methods are unsuccessful, more aggressive medical management (see section on medical management) should be considered. In dogs, as mentioned above, a constant rate infusion of glucagon can be considered to stabilize refractory patients. Postoperative pancreatitis and postoperative hyperglycemia appear to be uncommon complications in ferrets. Ferrets may be fed 24 hours after surgery. The prevalence of postoperative complications in cats undergoing surgery for insulinoma is not known, in part because pancreatitis is difficult to diagnose in cats due to variable clinical signs (see Chapter 138). The conservative postoperative management described earlier also is recommended for cats.
Treatment of Insulinoma in Dogs, Cats, and Ferrets
Diagnosis
Therapy
Emergency Treatment
Surgery
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


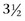 to 15 years old but is most common in dogs 8 to 12 years old. Insulinoma is rare in cats; five cats have been reported, ranging in age from 12 to 17 years. Insulinoma is common in domestic ferrets. The median age of ferrets with insulinoma has been reported to be 5 years, and the age range from 2 to 7 years. No sex predilection has been reported in dogs, but male ferrets seem to be affected more commonly than females.
to 15 years old but is most common in dogs 8 to 12 years old. Insulinoma is rare in cats; five cats have been reported, ranging in age from 12 to 17 years. Insulinoma is common in domestic ferrets. The median age of ferrets with insulinoma has been reported to be 5 years, and the age range from 2 to 7 years. No sex predilection has been reported in dogs, but male ferrets seem to be affected more commonly than females.