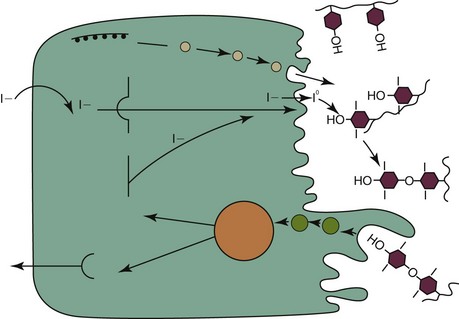
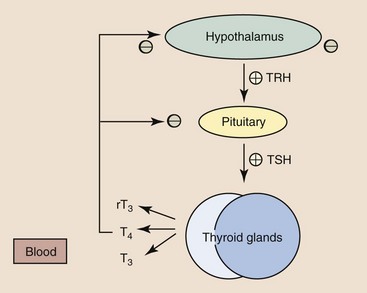
Web Chapter 20 Feline hyperthyroidism is the most common endocrine disease of geriatric cats, with a reported hospital prevalence of 3% (Edinboro et al, 2004), and is caused by autonomous secretion of thyroid hormones by the thyroid gland. Histopathology of affected thyroids usually reveals adenomatous hyperplasia or benign adenoma; however, rarely adenocarcinoma is diagnosed. Pathologic changes are bilateral in 70% of cats. Ectopic hyperplastic thyroid tissue may be present in the neck or thorax in up to 20% of cats. The thyroid hormones thyroxine (T4) and triiodothyronine (T3) are composed of iodine-containing amino acids synthesized in the thyroid gland (Web Figure 20-1). In blood, the majority of T4 and T3 are protein bound, with T4 more highly bound than T3. Only unbound thyroid hormone enters cells to produce a biologic effect and a negative feedback effect on the pituitary gland and hypothalamus. T3 enters cells more rapidly, has a more rapid onset of action, and is three to five times more potent than T4. Thyroid hormones bind to receptors in cellular nuclei; the hormone receptor complex binds to DNA and influences the expression of a variety of genes coding for regulatory enzymes. Thyroid hormones have a wide variety of physiologic effects; they increase the metabolic rate and oxygen consumption of most tissues, have positive inotropic and chronotropic effects on the heart, are catabolic for muscle and adipose tissue, stimulate erythropoiesis, and regulate both cholesterol synthesis and degradation. Thyroid hormone synthesis and secretion are regulated primarily by changes in the circulating concentration of pituitary thyrotropin (thyroid-stimulating hormone [TSH]) (Web Figure 20-2). Web Figure 20-1 Synthesis of thyroid hormones. The follicular cells of the thyroid gland concentrate iodide, which diffuses down a gradient into the colloid. Thyroglobulin is synthesized within thyroid follicular cells and secreted into the colloid. Iodide is oxidized and bound to tyrosine residues on the thyroglobulin molecule by thyroid peroxidase. Iodinated tyrosine residues (monoiodotyrosine [MIT] and diiodotyrosine [DIT]) within the thyroglobulin molecule then undergo oxidative condensation to form the iodothyronines (T3 and T4), which remain bound to thyroglobulin until secreted. Thyroglobulin is ingested by endocytosis from the colloid; the peptide bonds between the iodinated residues and the thyroglobulin are hydrolyzed; and MIT, DIT, T4, and T3 are released into the cytoplasm. MIT and DIT are deiodinated and the iodine is recycled, while T4 and T3 are released into the bloodstream. (From Mountcastle VB: Medical physiology, ed 14, vol 2, St Louis, 1980, Mosby.) Web Figure 20-2 Regulation of thyroid hormone concentrations. Thyroid hormone concentrations are controlled by the hypothalamic-pituitary-thyroid axis, which operates as a negative feedback loop. Thyroid-stimulating hormone (TSH) causes synthesis and release of T4 and lesser amounts of T3 from the thyroid gland. Intracellular T3, derived from deiodination of T4 within the pituitary gland, causes decreased TSH synthesis and secretion and is the main determinant of TSH concentration. Thyrotropin-releasing hormone (TRH), secreted by the hypothalamus, modulates TSH release from the pituitary gland. Increased thyroid hormone concentrations are also believed to decrease TRH synthesis and secretion. Hormones that inhibit TSH secretion include dopamine, somatostatin, serotonin, and glucocorticoids. TRH, prostaglandins, and α-adrenergic agonists increase TSH secretion. (From Scott-Moncrief JCR: Hypothyroidism. In Ettinger SJ, Feldman EC, editors: Textbook of internal medicine, ed 7, volume 2, St Louis, 2010, Saunders Elsevier, p 1752.) Feline hyperthyroidism is a disease of geriatric cats, with a mean age of 13 years (range 6 to 25 years). In addition to age, factors that have been identified as increasing the risk of feline hyperthyroidism include female sex, not being purebred, use of cat litter, contact with flea control products, and an increased consumption of canned cat food (Edinboro et al, 2004); ingestion of goitrogenic compounds, high intake of dietary soy, and decreased or increased intake of dietary iodine potentially play a role (Peterson et al, 2007). The cumulative effects of these exposures over many years may lead to mutations in thyroid follicular cells that ultimately result in autonomous thyroid hormone secretion. Mutations that have been identified in thyroid tissue from hyperthyroid cats include TSH receptor gene and G protein mutations. Diagnosis of hyperthyroidism can usually be confirmed by measurement of a single increased serum TT4 concentration. In cats with early hyperthyroidism or with concurrent nonthyroidal illness, the TT4 concentration may be within the upper half of the reference range. If the TT4 is high normal or borderline, the measurement should be repeated in 4 to 8 weeks or after concurrent diseases have been treated and resolved. Other diagnostic tests that can be used to confirm the diagnosis in cats with borderline TT4 concentrations include free T4 concentration, T3 suppression test, and nuclear scintigraphy. Radioisotope scanning using sodium pertechnetate is considered the most reliable diagnostic test for confirmation of hyperthyroidism in cats with concurrent illness (Shiel et al, 2007). The thioureylene drugs inhibit thyroid hormone synthesis by inhibiting thyroid peroxidase, an essential enzyme for thyroid hormone synthesis (see Web Figure 20-1). Thioureylenes do not influence iodide trapping by thyroidal follicular cells or release of preformed T4 and T3. Methimazole is the antithyroid drug licensed in North America, while carbimazole, a prodrug of methimazole, is available in Europe. The usual starting dose for methimazole is 2.5 mg per cat q8-12h PO and most cats respond clinically within 2 to 3 weeks of starting therapy. The maintenance dose should be titrated to maintain the TT4 within the reference range in general, and the lower half if possible; it is important to avoid both iatrogenic hypothyroidism, which is associated with shorter survival in azotemic cats, and mild hyperthyroidism (Williams, 2010). The final dose required for maintenance of euthyroidism ranges from 2.5 to 20 mg of methimazole per day. The most common adverse effects observed in up to 20% of cats include anorexia, vomiting, and lethargy. Transdermal administration of methimazole is associated with a lower risk of gastrointestinal side effects (Sartor et al, 2004). Other adverse effects include mild hematologic abnormalities such as leukopenia, lymphocytosis, and eosinophilia; less commonly severe neutropenia, thrombocytopenia, anemia, facial pruritus, toxic hepatopathy, and bleeding diatheses may occur, which should prompt immediate discontinuation of the drug. Most adverse effects occur within the first 3 months of treatment; thus cats treated with methimazole should have a CBC, platelet count, biochemical profile, and TT4 concentration evaluated every 2 weeks for the first 3 months of therapy.
Nutritional Management of Feline Hyperthyroidism
Thyroid Physiology
Epidemiology and Pathogenesis
Diagnosis
Treatment
Thioureylenes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


