CHAPTER 14 Cerebrospinal Fluid Analysis
COLLECTION TECHNIQUES
As mentioned earlier, it is important to emphasize that fluid obtained caudally to the lesion has more chances to be representative of the lesion rather than a fluid taken cranially to that lesion.1
SAMPLE PROCESSING
Because total protein and nucleated cell count are usually low compared with whole blood, a sample collected in a red-top (serum) tube is adequate for the great majority of CSF samples2 without risk of sample clotting. The cerebrospinal fluid is a fairly labile substance and, unless a stabilizing agent is added, is stable under refrigerated conditions for about 4 to 8 hours but, preferably should be analyzed within 30 minutes to 1 hour.2 Samples with higher protein content (> 50 mg/dl) are stable for a longer period than samples with lower protein content (< 50 mg/dl).3 A recent study3 showed a change in the percentage of large mononuclear cells after 2 hours, in small mononuclear cells after 12 hours, and in neutrophils after 24 hours in samples with no added stabilizing agents. Interestingly, the total cell count in those samples did not change significantly over a 24-hour period.3
Therefore, if a CSF sample cannot be analyzed within 1 hour of collection, the following procedure should be followed if there is sufficient sample (> 1 ml): the CSF should be separated into two aliquots: (1) one aliquot (unaltered) should be used for total protein and nucleated/red blood cell (RBC) count determination and (2) an aliquot containing 20% (volume:volume) fetal calf serum (FCS) or 10% (volume:volume) autologous serum should be used to perform the differential cell count. If the CSF sample is small (< 0.5 ml), adding hetastarch at a ratio of 1:1 should be done and all tests can be performed on the sample because adding hetastarch does not influence total protein. Keep in mind to correct the total cell count due to the dilution effect of the hetastarch.3,4
Cell Count
Usually, the CSF cell count is too low to be analyzed by automated machines such as the Cell-Dyn or Advia. Therefore, a cell count using a hemacytometer is still the gold standard for nucleated and red cell count.2 Some clinical pathologists prefer to stain the cells with new methylene blue (NMB) prior to counting, whereas others count unstained cells.
One technique to stain cells with NMB is to draw NMB into an unheparinized capillary tube until the capillary tube is about one-third full. Then, the stain is emptied from the tube and excess stain is blotted from the tip of the tube. The tube is then filled with CSF. The NMB adhered to the side of the tube stains the cells sufficiently to reveal cellular morphology. Due to the small amount of stain that is retained within the tube, this method does not influence cell count.3
Another technique is to draw a small amount of NMB into a capillary tube. The tube is tilted to allow the small amount of NMB to migrate to the middle of the tube, leaving an air pocket on either side of the NMB. A small amount of CSF is then drawn into the tube. The two liquids do not touch each other due to the presence of the air pocket. The capillary tube is then rocked back and forth several times so that the two columns of liquid move side to side, without touching. The tube is then allowed to sit for 10 minutes. The presence of the air pocket prevents the CSF and NMB from mixing together. Therefore, this technique does not influence the cell count.5
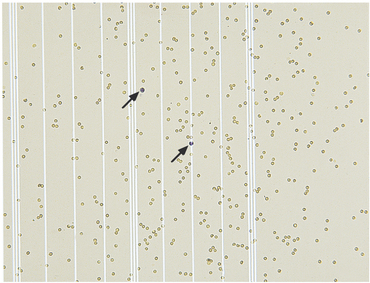
Cell counts (both nucleated and red cells) are performed by counting all cells (each cell type separately) in the nine large chambers of the hemacytometer. Both sides of the hemacytometer are counted and an average count is obtained by dividing the total of both sides by 2. Because the total area of one side of the hemacytometer is 0.9 mm3, multiplying the cell count by 1.1 gives the number of cells/μl2. Experience may be needed to differentiate small nucleated cells from RBCs: usually nucleated cells have a granular appearance, whereas RBCs have a smooth appearance or may be crenated. If NMB is used, leukocytes will have a blue appearance, whereas RBCs will be unstained (Figure 14-1).
Total Protein Determination
CSF fluid protein concentration is too low to be measured accurately by conventional biochemistry total protein techniques such as biuret or refractometer method. Instead, CSF total protein concentration is measured by using a Coomasie blue or pyrogallol red assay (Fisher Scientific, St-Laurent, QC). A crude method to determine the presence of excess protein in the clinical setting is to use urine chemstrip dipsticks (e.g., Multistix, Bayer Corporation, Elkhart, Ind.; Chemstrip 9, Roche Diagnostics, Laval, QC): the presence of a positive reaction indicates the presence of albumin because globulins are not detected by the dipsticks. Trace to 1+ protein is considered normal.6
The Pandy test is a screening test for the presence of immunoglobulin. It is performed by adding 2 or 3 drops of CSF to 1 ml of a 10% carbolic acid solution (10 mg of carbolic acid in 100 ml of distilled water2). Turbidity indicates the presence of globulins. The test is fairly sensitive with a sensitivity of approximately 50 mg/dl.5,7 Agarose gel protein electrophoresis can also be performed on CSF.8
NORMAL CSF
Normal CSF is a clear, colorless, water-like fluid. The presence of any color or turbidity must be considered abnormal (Table 14-1).
Type of cells
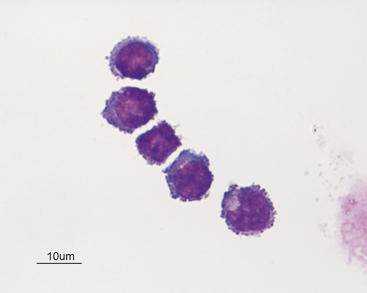
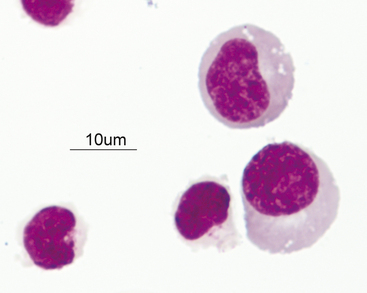
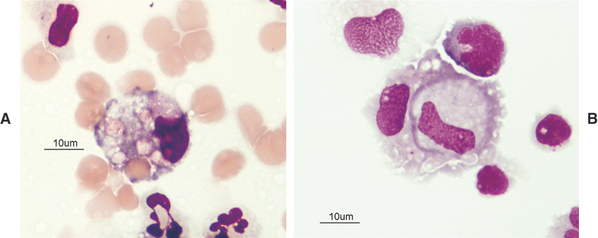
The great majority of cells in the CSF are mononuclear in nature with a mixture of small (Figures 14-2 and 14-3) and large (Figures 14-4 and 14-5) mononuclear cells. The presence of less than 10% nondegenerate neutrophils is considered normal by most authors12,13. Some studies have found up to 25% nondegenerate neutrophils in normal CSF.2 The term macrophage should be restricted to mononuclear cells showing evidence of material ingestion in their cytoplasm13 (Figure 14-6). If contamination from skin occurs, a few anucleate superficial keratinized cells may also be present (Figure 14-7).
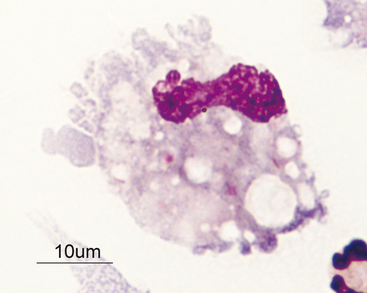
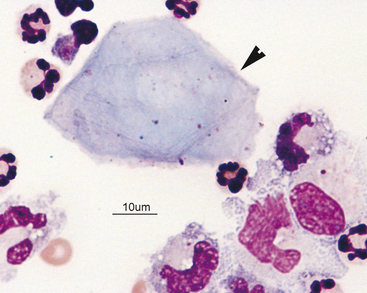
Figure 14-4 Large mononuclear cell with cytoplasmic vacuolation in a cerebrospinal fluid sample. (Wright-Giemsa stain.)
Other Parameters
INTERPRETATION OF ABNORMAL CSF
CSF cytology can be abnormal even though the cell count is within reference range.14 Therefore, even when CSF cell count and total protein values are within reference limits, a cytologic examination can be of value.
Blood Contamination and Hemorrhage
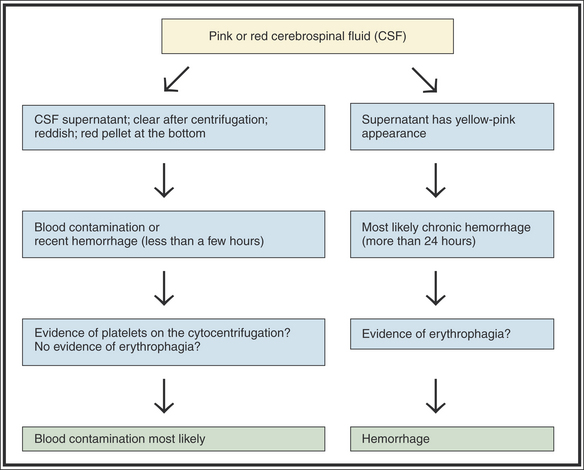
Due to the sometimes difficult nature of CSF collection, blood contamination is possible. For instance, in human medicine, one in five lumbar punctures are contaminated with blood.15 This blood contamination may result in a pink to frankly reddish appearance. When blood is present, the first thing to determine is if the blood present is contamination (iatrogenic) or secondary to hemorrhage in the CNS (Figure 14-8). If hemorrhage is older than 12 hours, evidence of erythrophagia by macrophages will be seen on cytology. Theoretically, the presence of fresh blood contamination should result in a combination of RBCs without erythrophagia and presence of platelets but, from our own experience, platelets may be difficult to visualize. Also, if hemorrhage of several days’ duration is present, the CSF supernatant should be xanthochromic (have a yellow to yellow-orange discoloration). However, some recent human studies suggest that the human eye cannot reliably detect xanthochromia in CSF. This means that hemoglobin breakdown products may be present in the CSF, but not detectable by the naked eye.16–17 Therefore, subarachnoid hemorrhage may be present in some instances, but a CSF discoloration will not be recognized. It can also be difficult to differentiate xanthochromia from icterus: a recent human study showed that about 80% of CSF samples with a significant amount of bilirubin appeared red instead of yellow macroscopically.17 Only spectrophotometry can differentiate xanthochromia from icterus adequately.
Effect of Blood Contamination on Total and Differential Cell Counts and Total Protein Concentration
The influence of blood contamination on total and differential cell counts and total protein concentration depends on the degree of blood contamination (Table 14-2). Some studies have shown that blood contamination yielding RBC counts of up to 15,000 RBC/μl does not influence either nucleated cell count or total protein concentration.18,19 Another study, however, found that an increase of 1 white blood cell (WBC)/100 RBCs could be found in some cases of blood contamination.13 Therefore, if the observed WBC count is higher than the predicted increase of 1 WBC/100 RBCs, the increased WBC count is likely real (i.e., it cannot be attributed to blood contamination alone). However, if the CSF WBC count is less than or equal to the predicted blood contamination value, the increase in WBCs might be real or might be secondary to blood contamination. If blood contamination of CSF appears likely from its gross appearance, the best approach is to try to repeat the CSF tap. As a general rule, RBC count > 3000/μl warrants collection of another sample (J. Parent, neurologist, Université de Montréal, Canada, personal communication).
Table 14-2 Influence of Blood Contamination on Nucleated Cell Count and Total Protein
| Red Blood Cell Count | Influence on Total Protein | Influence on Nucleated Cell Count |
|---|---|---|
| Less than 15,000/μl | No18,19 | No18,19 |
| Greater than 15,000/μl | Yes: overestimate18,19 | Yes: overestimate18,19 |
Use of Formulas to Establish Reliable Leukocyte Differential Counts in Cases of Blood Contamination
Several studies in human and veterinary medicine18,20,21 have shown that correction formulas do not reliably estimate “uncontaminated” WBC values in CSF. This is probably due to the fact that contamination of CSF samples by blood usually results from small meningial or spinal cord vessels that do not have the same proportion of WBCs/RBCs as large peripheral veins.18
CHANGES IN THE PERCENTAGE OF LEUKOCYTES WITHOUT PLEOCYTOSIS
Neutrophils
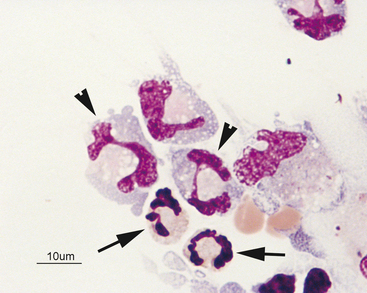
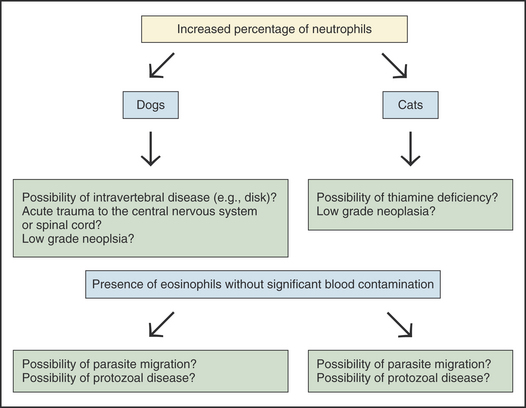
The presence of an increased percentage of neutrophils (10% to 20%) without a pleocytosis can be seen in several conditions (Figure 14-9). Acute trauma to the spinal cord, intravertebral disease,5 low-grade inflammation secondary to neoplasia, and thiamine deficiency (in cats)22 can result in an increased percentage of neutrophils without a pleocytosis.
ABNORMAL FINDINGS ASSOCIATED WITH INCREASED CELL COUNT (PLEOCYTOSIS)
Pleocytosis refers to an elevated nucleated cell count in the CSF. The magnitude of the pleocytosis and the type of cells present are both important in recognizing certain disease processes. For the purposes of this discussion, mild will refer to counts higher than reference limits but less than 25 cells/μl, moderate will refer to cell counts between 26 and 100 cells/μl, and marked will refer to cell counts in excess of 100 cells/μl.5