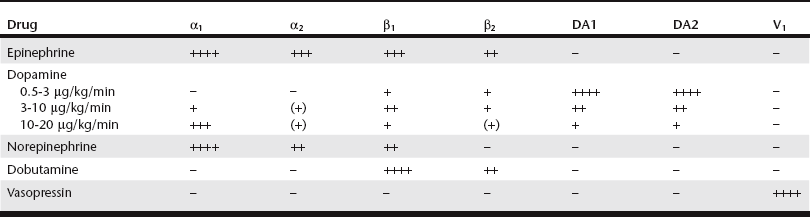
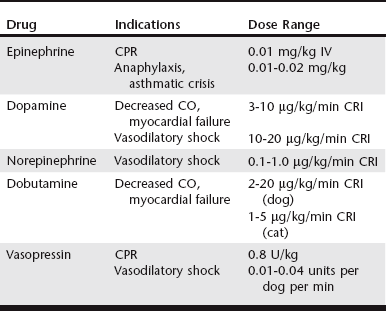
Chapter 3 Given the varied effects and tissue beds that are subject to sympathetic stimulation, there is significant potential for therapeutic manipulation through administration of exogenous catecholamines. In critical care patients these medications are primarily used to augment catecholamine effects on the cardiovascular system, especially when the endogenous response might be insufficient or become exhausted. Determining which catecholamine to use is largely based on its relative receptor activities (Table 3-1), the clinical scenario in which intervention is needed (Table 3-2), and the potential for adverse effects. The primary beneficial effect of EPI in cardiopulmonary resuscitation (CPR) is α-induced vasoconstriction (and resultant increase in diastolic pressure) rather than any direct cardiac effects from β stimulation. Coronary circulation is an essential component of successful resuscitation, and diastolic pressure is a major determinant for coronary perfusion pressure. In addition, EPI has beneficial effects on cerebral perfusion and oxygen delivery. Despite these potential benefits, available evidence shows no clear survival benefit with administration of EPI in CPR (Neumar et al, 2010). However, EPI remains the drug of choice in CPR because it has been shown to improve rates of return of spontaneous circulation (ROSC). Ideal dosing has been a subject of some debate. Although it has increased α1 stimulation, “high-dose” EPI (0.1 mg/kg) has not proved any more effective in achieving ROSC than “low-dose” EPI (0.01 mg/kg) in most clinical circumstances of arrest. Also, with “low-dose” EPI some of the adverse effects of EPI are avoided in the postresuscitation period, especially if repeated doses are needed, and this “low-dose” approach currently is recommended in CPR (Neumar et al, 2010). One must be mindful of the concentration of epinephrine used (1 : 1000 vs 1 : 10,000). For “low-dose” EPI, standard dosing with 1 : 10,000 is 0.1 ml/kg (or 1 ml/10 kg). If only 1 : 1000 is available, it should be diluted 1 : 10 in sterile water and then administered as above. This can be repeated every 3 to 5 minutes (after each one to two cycles of compressions) until ROSC or cessation of CPR. When administered via the endotracheal route if intravenous (IV) access is unavailable, doses administered during CPR should be doubled. Another potential application of EPI is in the treatment of anaphylaxis/anaphylactic shock. The combination of vasoconstriction and bronchodilation makes EPI, along with IV fluids and oxygen therapy, ideally suited to help reverse the major life-threatening aspects of anaphylaxis. In addition, EPI serves to decrease production and release of mediators involved in the pathogenesis of anaphylaxis. Although there is some evidence of improved outcomes with a constant rate infusion (CRI) of EPI (Mink et al, 2004), bolus dosing of 0.01 to 0.02 mg/kg IV (potentially repeated after 15 to 20 minutes) is still currently recommended. EPI also may be beneficial as a bronchodilator for cats in asthmatic crisis, dosed at 0.01 to 0.02 mg/kg IV or subcutaneously (SC).
Catecholamines in the Critical Care Patient
Specific Catecholamines
Epinephrine
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree