Cardiorespiratory Disease
11.1 Anatomy and physiology of the respiratory system
Rabbits have sensitive nostrils and a good sense of smell. There are 20–25 vibrissae in each upper lip. In healthy rabbits, the nostrils constantly twitch at a rate of 2–120 times per minute, unless the rabbit is at rest (Brewer and Cruise, 1994) or is unwell. The nasal cavity is lined with a protective layer of mucus that entraps foreign particles and bacteria. The mucus also prevents water loss and enhances the sense of smell. The nasal glands secrete serous fluid into the nasal cavity. In the rabbit, there is glandular tissue along the nasal septum and a cluster of glands, collectively known as the lateral nasal gland, occupy the entire wall between the nasal cavity and maxillary sinus (Bojsen-Moller, 1964). The function of these nasal glands is to moisten inspired air, which has a role in thermoregulation. The position of the conchal and maxillary sinuses and the structures of the nasal cavity are illustrated in Figures 1.18 and 5.1. There is no frontal sinus.
The oropharynx is narrow and the base of the tongue is large in rabbits. The glottis is small. Breathing takes place through the nostrils; the epiglottis is engaged with the soft palate, making the rabbit an obligate nasal breather. Mouth breathing only occurs during severe respiratory distress.
Each lung is divided into cranial, middle and caudal lobes and there is an accessory lobe on the right lung. Respiratory movement in rabbits is mainly diaphragmatic rather than due to the action of the intercostal muscles. This means that respiration can be restricted when there is increased intra-abdominal pressure. The thoracic cavity is small and the thymus, which in contrast with other species does not regress, but remains large throughout life, occupies the anterior ventral thoracic cavity (see Figure 11.7).
11.2 Respiratory diseases
11.2.1 Pasteurellosis
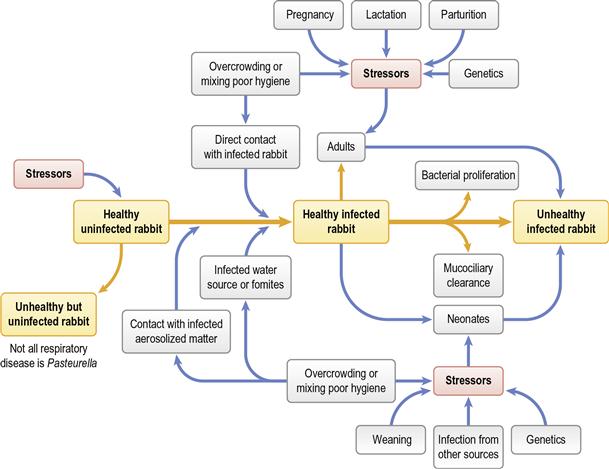
Pasteurella multocida is associated with a number of diseases of rabbits (see Section 14.5.1). Pasteurellosis is not a recognized problem in wild rabbits but is a serious disease in colonies of commercial or laboratory rabbits. In pet rabbits, although P. multocida is found as an opportunist pathogen in many secondary infections, primary pasteurellosis is uncommon. It is usually encountered in situations where there has been increased stress and an increased number of interactions with other rabbits: for example, the newly acquired rabbit that has recently been bought from a breeder or pet shop. Respiratory disease is the most common manifestation. Acute infections and septicaemia occur, especially in young animals, but chronic, insidious recurrent infections are more common in the adult pet rabbit. Rhinitis, conjunctivitis, nasolacrimal duct infections, otitis media, tracheitis and bronchopneumonia can all be caused by P. multocida; however, it is wise to remember that other organisms can be involved. The bacteria can spread to other sites from the nasal cavity where it can reside as a commensal organism. Infection often persists despite mucosal and humoral antibody responses in addition to effusive neutrophilic exudation. The deleterious effect of pasteurellosis on laboratory colonies of rabbits and interference with experimental procedures has resulted in the evolution of expensive ‘pasteurella-free’ rabbits for use in research. The epidemiology of pasteurellosis is discussed in Section 14.5.1. See Figure 11.1 for the many predisposing factors that trigger disease.
11.2.2 Respiratory disease due to pasteurellosis
Pet rabbits are often already infected with P. multocida when they are purchased from a pet shop or breeder. Lu et al. (1983) surveyed the capsular and somatic serotypes of P. multocida found in healthy and diseased rabbits and concluded that while several major serotypes can be differentiated, one (serotype 12A) was predominant in both healthy and diseased individuals. The development of rhinitis and other respiratory tract problems in the newly acquired young rabbit is likely to be due to pasteurellosis. In the older animal, stress or poor husbandry can result in a flare-up of a latent infection. The possibility of disease being due to pasteurellosis does not replace the need for diagnostic evaluation. Poor air quality, caused by high ammonia levels or dusty hay, irritates the respiratory tract and predisposes secondary infection. Ventilation and good air quality are important in disease prevention. Pasteurellosis can be spread between animals and the disease is endemic in most breeding establishments. It often causes problems in premises such as sanctuaries, where several animals are housed in close proximity. A distance of greater than 1.8 m (6 feet) or ‘sneezing distance’ is needed to control the spread of infection between individuals (Whittaker, 1989).
11.2.3 Rhinitis (‘snuffles’)
Repetitive sneezing and upper respiratory tract noise is a feature of rhinitis. Rhinitis and sinusitis can be manifestations of pasteurellosis, although other organisms such as staphylococci or Bordetella can also be involved. The differential diagnosis of upper respiratory tract disease in rabbits includes nasal foreign bodies and periapical abscesses of the maxillary incisors or premolars. Both these conditions are common in the pet rabbit, so it cannot be assumed that all rabbits with a purulent nasal discharge are suffering from pasteurellosis or that all cases of ‘snuffles’ are due to infectious agents.
In the initial stages of pasteurellosis, the nasal discharge is serous and the condition is responsive to antibiotic therapy. In advanced cases, the nasal discharge is thick, yellow and viscid. Copious amounts of mucopurulent material can be discharged from the nostrils and form crusts on the surrounding skin. Affected rabbits wipe the purulent discharges from their nose with their forepaws, which become matted and discoloured. Coughing is not as common as sneezing and snorting. Respiratory noises may be audible to the owner who may think their rabbit is ‘wheezing’. Anorexia can occur, perhaps due to a reduced sense of smell or because it is difficult to chew and breathe at the same time. Grooming difficulties occur because the rabbit finds it difficult to breathe and groom simultaneously. Response to antibiotic therapy is poor in advanced cases and relapse is common. Post-mortem examination of the sinuses and nasal passages of rabbits with chronic rhinitis shows why these cases are so difficult to treat. The nasal cavity is filled with pus, which can spread into the paranasal sinuses (see Figure 5.1 and Figure 11.2). The pus becomes thick and inspissated. There is ulceration of the mucous membranes and osteomyelitis of the turbinates, causing severe atrophy and erosion (Deeb, 1997). The presence of pus in the nasal cavity impedes gas exchange and causes physical discomfort and irritation.
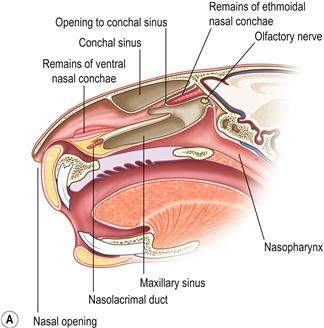
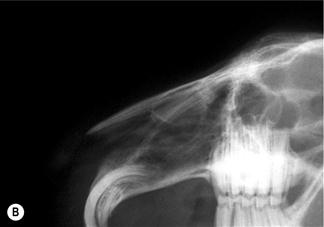
Figure 11.2 (A) Sagittal section through the head to show the position of the paranasal sinuses.
This figure was drawn from a post-mortem specimen of a sagittal section through a decalcified head. The nasal conchae have been removed. There are two paranasal sinuses in rabbits: the conchal sinus and the maxillary sinus (sometimes called the maxillary recess). There is no frontal sinus. Both the conchal and the maxillary sinus form blind cavities. At the cranial end of each sinus there is a single opening into the nasal passage. The structures of the nasal cavity are illustrated in Figure 1.20. The position of the paranasal sinuses is also illustrated in Figure 5.1.(B) Chronic infection of the conchal sinus.
This figure is a lateral view of the nasal cavity of an 18-month-old dwarf lop female rabbit with chronic rhinitis. The rabbit had started sneezing and developed a nasal discharge shortly after she was purchased at 10 weeks of age. Antibiotic therapy and mucolytic therapy failed to cure the condition although the symptoms were temporarily alleviated. Radiology shows increased radiopacity of the conchal sinus, indicating the presence of infection. There is erosion of the turbinates. The conchae and ethmoturbinates are not visible on the radiograph. Rhinitis was due to infection and not related to dental disease.
11.2.3.1 Differential diagnosis of rhinitis
The clinical history can be very suggestive of pasteurellosis. Young rabbits that have recently been stressed by weaning, change in routine and transport are often exposed to infection at the breeding establishment where they originated. Rabbits housed with several others in sheds and outhouses are susceptible. In older, individual pet rabbits, bacterial infection is less likely to be a cause of rhinitis than dental disease, which is common. Nasal foreign bodies can cause rhinitis (see Section 11.3). Myxomatosis is another possible cause of rhinitis. Myxomatosis in rabbit colonies can present as rhinitis in association with ocular discharge. Aerosol infection is more likely to give respiratory tract signs than insect spread (see Section 14.6.1). Myxomatosis is progressive and almost invariably fatal.
Bacteriology can be used to identify bacteria present in the nasal passages of rabbits with rhinitis and to ascertain antibiotic sensitivity. The rabbit’s nose is sensitive and it can be difficult to insert the swab deep into the nasal passages in the conscious animal. Sedation or anaesthesia is usually required to take a deep nasal swab for culture. Culture results do not necessarily illustrate the organism causing the clinical signs; it merely gives an overview of the organisms present. Caution is therefore necessary in their interpretation. Depending on the method of collection of swab samples for culture, contaminants can be present, presenting a false result. Common causes of inaccurate results are failure to clean the external nares prior to sample collection, and failure to insert the swab deeply enough into to nasal cavity. False-positive and -negative results can occur on bacterial culture due to inaccuracies in various laboratory methods of bacterial strain identification.
Underlying dental problems can be diagnosed by visual examination and by radiography (see Section 5.6 and Figure 11.2B). The structures of the nose and teeth can be assessed on skull radiographs. The paranasal sinuses can be identified (see Figures 5.10 and 11.2) and abnormalities may be detected radiologically. Opacity of the conchal sinus indicates the presence of exudate (see Figure 11.2B). Erosion of the ethmoturbinates can be seen on a well-exposed radiograph. However, the presence of P. multocida or other bacterial infection and erosion of the turbinates does not rule out the possibility of an underlying foreign body or perapical abscess. Large abscesses or rhinoliths can form in the nasal passages because of tooth root infection (see Figure 5.12).
11.2.4 Otitis media
Pasteurella multocida primarily resides in the nasal cavity but can spread via the eustachian tube to the tympanic bulla and affect the middle ear (see Figure 11.3). Infection can spread further to affect the inner ear and vestibular apparatus or track through the acoustic meatus and along the vestibulocochlear nerve. On post-mortem examination abscesses can be found in the cranial cavity.
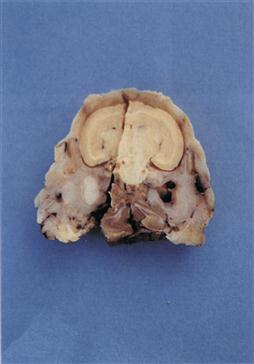
Figure 11.3 Pus in tympanic bulla.
A cross-section of a decalcified head is shown. There is also a sagittal section through the specimen. The skull is from a Netherland dwarf male rabbit that developed a head tilt. Despite an initial response to antibiotic therapy, the rabbit relapsed and was euthanized. The skull has been sectioned at the level of the tympanic bullae. The left tympanic bulla is filled with pus.
It is not easy to diagnose otitis media in the live rabbit and in many cases clinical examination and routine imaging are not sufficient. Hammond et al. (2010) demonstrated that plain radiography of the rabbit skull had a specificity and sensitivity for finding fluids similar to that of cat and dog radiography, and confirmed that the dorsoventral view was the most consistently useful one. The presence of exudate in the external ear canal does not signify the presence of otitis media. In many pet rabbits, especially lop-eared breeds, it is difficult to visualize the tympanic membrane due to the presence of waxy ear secretion. Post-mortem examination of the ear canal of pet rabbits often reveals the presence of inspissated pus that occludes the external ear canal. The presence of purulent material in the external ear canal does not necessarily signify the presence of pus in the tympanic bulla. Neither does pus in the tympanic bulla always cause otitis interna and vestibular symptoms (see Section 10.4.3). Radiological changes can often be seen as an incidental finding on skull radiographs (see Figure 11.4). It seems likely that rabbits with pus in the external ear canal and/or the tympanic bulla will have impaired hearing, although they may appear clinically normal. Some observant owners can detect hearing deficits in their pets and aggression in rabbits has been attributed to deafness when rabbits have been startled by the unheard approach of their owners. Computed tomography scanning or ultrasonographical examination of the area (King et al., 2007) will give more useful and sensitive information.
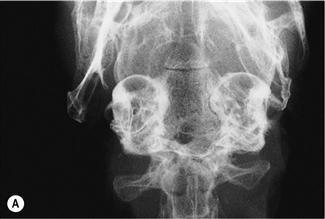
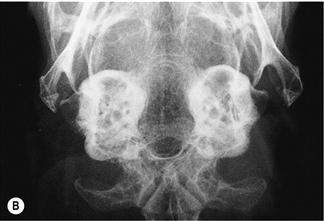
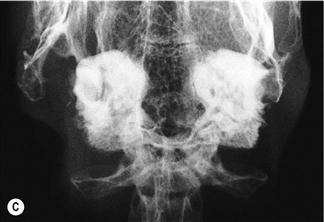
Figure 11.4 Radiographic changes of the tympanic bulla.
(A)–(C) are dorsoventral views of the caudal area of the skull to show progressive changes of the tympanic bullae from normal (A) to severe (C); (B) is an incidental finding on a rabbit that was radiographed to investigate his dental disease; and (C) is of a 3-year-old French lop male that was rescued by the RSPCA from a small shed containing between 20 and 30 rabbits. Upper respiratory problems were endemic. The rabbit appeared totally deaf and was ataxic. He was quiet and subdued. He was euthanized due to dental problems. Post-mortem examination showed pus in both tympanic bullae.
In a study by Flatt et al. (1977), otitis media was found in 4% of 2001 young rabbits and 32% of adults slaughtered for human consumption. The animals appeared clinically healthy on ante-mortem inspection. Gross lesions included the presence of white tenacious exudate filling the tympanic bulla. The mucous membrane lining the bulla was thickened, translucent and discoloured. The eustachian tube was dilated and filled with pus. Microscopic lesions consisted of an accumulation of heterophils in the lumen of the tympanic bulla and in the mucosa and underlying periosteum. In some of the affected ears, the simple squamous epithelium over the tympanic membrane and auditory ossicles had undergone squamous metaplasia with necrosis of the mucosa in severe cases. In the rabbits with periosteal changes, the thickened periosteum was infiltrated by a variable number of heterophils, plasma cells and lymphocytes. Occasionally, granulation tissue was present. In some cases, the tympanic membrane was ruptured and a suppurative exudate was present in the tympanic cavity and the external auditory canal.
11.2.5 Pneumonia
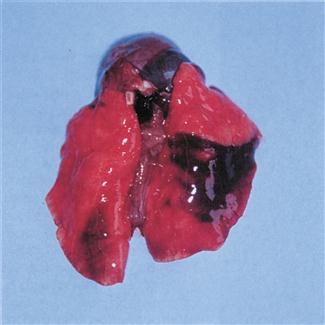
Septicaemia and acute suppurative pneumonia can be caused by P. multocida. Infection can be peracute and cause rapid death (see Figure 11.5). Chronic pneumonia and abscesses in the thoracic cavity also occur. Poor exercise tolerance and increased respiratory rate may not be obvious to owners of pet rabbits that are confined to hutches or small runs with no opportunity to exercise. These individuals pose a poor anaesthetic risk due to poor gaseous exchange in consolidated lungs.
11.2.6 Treatment of pasteurellosis
Pasteurellosis is a difficult condition to cure. Acute upper or lower respiratory infections can be responsive to prompt antibiotic and non-steroidal anti-inflammatory medication. An antibiotic that is unlikely to cause diarrhoea but is effective against P. multocida should be selected. Examples include enrofloxacin, trimethoprim sulpha combinations, tetracyclines, parenteral cephalexin or penicillin. In vitro, most rabbit isolates of P. multocida are susceptible to penicillin, chloramphenicol, tetracycline, erythromycin, novobiocin and nitrofurans with a variable susceptibility to streptomycin, kanamycin, neomycin and sulphonamides. Pasteurella multocida is usually resistant to clindamycin and lincomycin (Manning et al., 1989). More recently Sellyei et al. (2009) demonstrated the increasing resistance of P. multocida isolates from swine and poultry to certain antibiotics (namely, sulphonamides, tetracyclines and aminoglycosides); however, they found third-generation quinolones such as enrofloxacin to be effective. The tendency for the fluoroquinolones to concentrate in the mucosa of the maxillary sinus at levels greater than that found in the blood (Dan et al., 1989) makes this drug a good first choice for treating Pasteurella. Penicillin has been used widely to treat rhinitis in laboratory rabbits (Gaertner, 1991). Concurrent infection with other pathogens such as Bordetella bronchiseptica can affect the response to therapy. Tilmicosin is an effective antibiotic in the treatment of acute pasteurellosis in sheep and has been used to treat rabbits despite the possibility of a fatal adverse reaction to the drug.
Long-term or periodic courses of antibiotic can be given to control long-standing infections although they are unlikely to be curative. Antibiotics can also be introduced directly into the nose. Some rabbits will tolerate this procedure. Gentamicin is available as an ophthalmic preparation (Tiacil, Virbac) that can be used as nose drops. Purulent exudate needs to be removed before the drops are instilled.
Chronic pasteurellosis is manifested by the presence of copious quantities of thick, viscid, mucopurulent material that presents a physical barrier to medication. The pus is often in inaccessible sites such as the nasal passages, paranasal sinuses, tympanic bullae or even the brain. Surgery, such as trephination, to remove the pus, provide drainage and create a route for local medication, is possible. Bulla osteotomy has been suggested as a treatment for rabbits with severe, refractory, chronic otitis media in association with vestibular signs such as head tilt and anorexia (Redrobe, 2000). Two surgical techniques have been described (Redrobe, 2000; Swindle and Shealy, 1996). Chow et al. (2009) and Chow (2011) describe in detail the technique for ventral bulla osteotomy in the rabbit and include a period of follow-up, with encouraging results. This experience has been replicated by the staff of the Exotics Service at the University of Edinburgh. Trephination of sinuses has been widely described in rabbits (Gilbert et al., 2004; Scharf et al., 1994; Watanabe et al., 1999) as they are used as models of maxillary sinusitis in humans; however, clinical reports are less common but the procedure appears to be well tolerated (Kelleher, 2008). Trephination rarely provides freedom from clinical signs of chronic sinusitis in cats.
The chances of successful treatment of pasteurellosis are greater in sites where the pus can be removed, e.g., by flushing an infected nasolacrimal duct or removing an abscess or infected organ such as a uterus or testicle. Dacryocystitis, facial abscesses or purulent nasal discharges are often associated with underlying dental disease that needs to be addressed if there is to be any hope of success in treating the secondary Pasteurella infection (see Sections 5.5.1 and 6.1).
In cases of rhinitis, it is important to establish adequate systemic hydration by ensuring adequate fluid intake. Dehydration leads to dry airways, resulting in increased viscosity of secretions, decreased ciliary function, inflammation and degeneration of the mucosa. The inclusion of fresh leafy vegetables in the diet can increase a rabbit’s fluid intake, particularly if they are fed wet (water is left on the vegetables after washing). Water can be added to inspired gases by the use of humidifiers or placing the rabbit in a steamed-up room such as a bathroom, although care must be taken to ensure the rabbit does not overheat. Nebulization is sometimes used as a method of introducing antibiotic, decongestants and other agents directly into the respiratory tract and to loosen secretions and bring relief. Nebulization introduces charged particles into the respiratory tract as an aerosol. In other species, nebulization is used to treat lower respiratory tract disease. Medication introduced by nebulization is unlikely to reach the tympanic bullae or paranasal sinuses or to penetrate thick mucopurulent exudate in rabbits. Mucolytic agents such as bromhexine or N-acetyl-cysteine have been recommended for nebulization in rabbits with rhinitis (Meredith, 2000). In other species, N-acetyl-cysteine is irritating to mucosal surfaces and can inactivate certain antibiotics when it is mixed with them (McKiernan, 1983). Systemic bromhexine (Bisolvon, Boehringer Ingelheim) can be used as a mucolytic in rabbits. In cattle and pigs, when bromhexine is administered simultaneously with oxytetracycline, the antibiotic in the bronchial mucus is considerably increased (product datasheet).
Occasionally, owners administer human decongestants to their rabbits. There is no proven efficacy in the use of such products in the treatment of ‘snuffles’. Oxymetazoline is a common topical nasal decongestant that has been investigated in experimentally induced infections of the maxillary sinus in rabbits (Bende et al., 1996). Paradoxically, a higher degree of inflammation was found in the oxymetazoline-treated sinuses. The authors concluded that oxymetazoline nose drops interfere with the normal defence mechanisms, possibly by a decrease in mucosal blood flow.
Topical application of fluoroquinolones to the surface of the eye has been shown to result in concentrations of drug greater than the MIC90 for most ocular pathogens for 6 or more hours (Dan et al., 1989; Green et al., 1996; Hendrix and Cox, 2008). The use of ciprofloxacin eye drops has been suggested for adjunctive treatment of rhinitis and sinusitis in rabbits (Carpenter, 2007), because the tear film drains into the nasal sinuses. The current author (MJV) has found this very useful clinically as part of a multi-therapeutic approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree