Chapter 22
Cardiopulmonary cerebral resuscitation (CPCR)
Jennifer G. Adams
Causes of cardiac arrest/risk factors for anaesthetic mortality
Basic cardiac life support ‘A-B-C’
‘A & B’ – airway and breathing
‘C’ – Circulation – cardiac compressions
‘D’ – advanced cardiac life support
Cardiac arrest rhythms and electrical shock
History and introduction
Organized efforts to promote resuscitation began centuries ago. The Dutch Humane Society and the Royal Humane Society were both founded in the 1700s in Amsterdam and London, respectively, after realizing that drowned or otherwise injured individuals could be revived (Sternbach et al., 2005; Cooper et al., 2006). These groups sought to enlighten the public as to this phenomenon and to teach the techniques necessary for resuscitation. By the 1950s, concerted efforts to educate the lay public in cardiopulmonary resuscitation (CPR) techniques were underway. Currently, CPR classes are offered by numerous local and national agencies or institutions and many, many lay people have been trained in resuscitation techniques of humans. With the development of the automated external defibrillator (AED), ‘bystander CPR’ has become a significant factor in saving the lives of humans in cardiac arrest. In fact, AEDs are readily available in many public places, for example, the Hartsfield-Jackson International Airport in Atlanta, Georgia has more than 200 portable AEDs positioned throughout the facility (www.atlantaairport.com). CPR techniques for animals are published and available to pet owners in print form and on the Internet at numerous websites sponsored by pet rescue groups, pet care organizations, and veterinarians. Classes in ‘pet CPR’ are also available from many sources.
A report by Beecher and Todd published in 1954 reported an anaesthetic mortality of 1 : 2680 in humans following examination of the anaesthetic records of almost 600 000 cases at 10 institutions over a 5-year period. Mortality associated with anaesthesia has since greatly decreased in humans; one report states that death is expected to occur no more often than 1 in 200 200 cases (0.0005%) involving healthy patients (Eichhorn, 1989); another reports 1 : 13 000 (0.008%) when all cases are considered (Lagasse, 2002). Veterinary anaesthetic mortality rates have also improved since the 1950s, but are still much higher than humans. Rates of 1 : 555 (0.18%) in healthy dogs, 1 : 385 (0.26 %) in healthy cats (Brodbelt et al., 2008), and 1 : 111 (0.9%) (Johnston et al., 2002) to 1 : 1250 (0.08%) (Bidwell et al., 2007) in horses (excluding colic surgery) are reported most recently. In spite of advances in techniques and treatments and widespread training in cardiopulmonary cerebral resuscitation (CPCR), the overall success rate following CPCR attempts is still poor in both humans and animals; 17.6% of inpatients and 6.4% of outpatients survived cardiac arrest when the results of three human studies were combined (Cooper et al., 2006). A recent study of survival following CPCR at a veterinary teaching hospital reported that no patients requiring CPCR at admission survived. It also found that only 6% (12/204) of all dogs and cats experiencing in-hospital cardiopulmonary arrest (CPA) survived to discharge (Hofmeister et al., 2009). This is similar to an earlier review of CPA in hospitalized patients where only 4.1% of dogs and 9.6% of cats survived to discharge (Wingfield & Van Pelt, 1992). When patients experience cardiac arrest under anaesthesia, the % return of spontaneous circulation (ROSC) and survival to discharge from hospital is usually much higher. In Hofmeister et al. (2009), nine of the 12 (75%) animals that survived were anaesthetized at the time CPA occurred; the only survivors of CPA (4/135) in a 4-year period at another veterinary teaching hospital had been under anaesthesia when arrest occurred (Kass & Haskins, 1992). Even though the outcome of anaesthesia-related CPA may be better, successful CPCR is certainly not always likely; thus prevention of CPA is the best course.
Cerebral resuscitation
Outcome measures in CPR studies originally focused on the return of spontaneous circulation and hospital discharge of patients. However, the number of patients with ROSC is much greater than those that actually survive to be discharged with normal or minimal neurological dysfunction. Hence, more emphasis is now placed on neurological outcome, long-term survival, and quality of life post cardiac arrest. The acronym was changed to CPCR to reflect this emphasis. Return of effective cerebral circulation begins with re-establishment of cardiopulmonary function via the basic elements of CPR, such as timely recognition of arrest, immediate institution of effective CPR resulting in ROSC, and prevention of systemic complications in the post-arrest period. Attention to specific techniques such as using continuous cardiac compressions and avoiding pauses may allow improved neurological outcome. Optimal post-resuscitation therapy is important to minimize the damage that occurs following reperfusion of the ischaemic brain. Homeostatic parameters such as blood pressure, acid–base status, electrolytes, blood gases, glucose, should be maintained in the normal range. Treatment to decrease neuronal death and improve cerebral function such as therapeutic hypothermia has already been adopted; others are now being examined, including neuronal cell growth promotion, inhibition of reperfusion injury and neuronal apoptosis. Clinical signs, electrophysiological testing, diagnostic imaging, and measurement of neuron-specific proteins in serum are used to evaluate cerebral function and estimate a prognosis following resuscitation (Schneider et al., 2009).
The science behind CPCR techniques has been periodically evaluated by The International Liaison Committee on Resuscitation (ILCOR). This group was formed in 1992 to represent a consortium of scientists working in the resuscitation and critical care fields. It presently includes groups from the six major continents. The purpose of ILCOR is to identify information relevant to resuscitation, emergency and critical care medicine, perform an evidence-based review of this information, and develop a consensus of guidelines based on the strength of the scientific evidence. In cooperation with the American Heart Association, ILCOR produced the first International CPR Guidelines in 2000 and an International Consensus on CPR and Emergency and Critical Care (ECC) Science with Treatment Recommendations in 2005, published in the journals Circulation and Resuscitation. The group currently meets biannually and plans to publish updates every 5 years. The most recent reviews were published in October and November 2010, and can be obtained at the ILCOR website, www.ilcor.org/en/home/. Although the purpose of ILCOR is to define best practices for humans, much of the research is performed in anaesthetized animals, especially dogs and swine, and is potentially applicable to veterinary medicine. Since animals used in research are generally healthy, some differences will emerge when study findings are used in clinical populations. Plunkett and McMichael (2008) published a very thorough update of CPCR in small animals based on the 2005 ILCOR guidelines.
Most recently, an extensive review of the literature as it specifically pertains to resuscitation of small animal veterinary patients was conducted by a group of 101 veterinarians, specialists in internal medicine, anaesthesia, and critical care. The investigation was conducted very similarly to the protocol used by the ILCOR group. They developed topics pertinent to the performance of CPCR in animals and explored the literature for evidence. From their efforts, called the Reassessment Campaign on Veterinary Resuscitation (RECOVER) initiative, a set of consensus guidelines for CPCR in dogs and cats was published in a supplement to the Journal of Veterinary Emergency and Critical Care (2012) (see suggested reading) and is available at www.veccs.org or www.onlinelibrary.wiley.com.
Prior to anaesthesia
The prevention of anaesthetic complications including cardiac arrest begins prior to the anaesthetic episode. A thorough preanaesthetic evaluation including physical examination, laboratory evaluation, and diagnostics appropriate to the patient and problems present is vital to identify pertinent risk factors. A discussion of the anaesthetic plan and potential complications for each case should take place preoperatively between all members of the anaesthesia staff. All equipment, drugs, and supplies required for treatment of anaesthetic complications should be conveniently within reach of the anaesthetized patient at all times. Those specific to CPCR should be immediately available; standard doses of epinephrine and atropine should be calculated and even drawn up prior to induction for specific cases or patients (i.e. birds, small mammals, very small dogs and cats, severely ill patients). Reference charts with the CPCR protocol and emergency drug dosages should be posted in anaesthesia and surgery areas. All staff potentially involved in performance of CPCR should have formal training. Since cardiac arrest is not a common event, practice sessions of CPCR techniques should be performed on a regular basis. Review of the team’s performance following resuscitation events can identify deficiencies and improve outcome of subsequent cases. Finally, phone numbers for immediate contact with owners should be available and resuscitation status should be determined from discussions with the owner at admission. This should be documented in the patient record, and posted on the patient’s cage/stall. Suggested categories are: (1) do not attempt resuscitation (DNAR); (2) resuscitation efforts limited to external compressions and medical therapy; and (3) unlimited efforts including internal cardiac compressions (Rieser, 2000).
Causes of cardiac arrest/risk factors for anaesthetic mortality
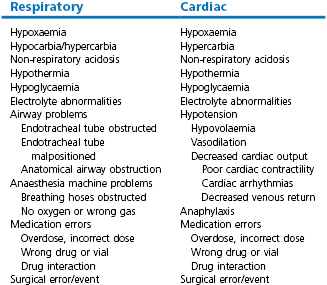
The immediate cause of cardiac arrest under anaesthesia is always associated with respiratory and/or cardiac insufficiency, as lack of oxygen rapidly results in dysfunction of brain and myocardial cells. Hypoxaemia, hypoventilation, hypotension, hypovolaemia, cardiac arrhythmia, hypothermia, anaesthesia machine or equipment malfunction, drug interactions or overdose, and anaphylaxis are the most common precipitating causes (Table 22.1). These can be associated with or directly the result of the patient’s condition, anaesthesia, or surgery. Occasionally, events occur that are not preventable. The classic clinical signs of cardiac arrest are loss of a palpable pulse, lack of heart sounds with auscultation, cyanotic mucous membranes (except in sudden arrest where mucous membranes remain pink for a few minutes), apnoea, pupillary dilation, and lack of bleeding from surgical incisions. Other signs include very slow capillary refill time, and loss of the palpebral reflex. Although it often seems to happen quite suddenly, some cases of arrest involve more than one problem, developing over the course of the anaesthetic episode.
Sudden changes in vital signs may not always occur prior to an arrest. Clinical signs that should prompt the anaesthetist to be suspicious of an impending arrest include gradually decreasing or increasing heart rate, continuing change in the mucous membrane colour, capillary refill time, pupil size, or ocular responses, frequent premature contractions, frequent atrioventricular heart block, decreasing end-tidal CO2 levels, decreasing or increasing temperature, slower respiratory rates, and very irregular or gasping respiratory patterns.
Hypoventilation and the resulting hypercarbia produce clinical signs that may mimic a ‘light’ depth of anaesthesia as rapid deep breaths may occur (Kussmaul respiratory pattern); limb motion, muscular twitching, tachycardia, and hypertension may also be seen. This may mislead the anaesthetist into giving more drugs, further exacerbating respiratory insufficiency. Unusual respiratory patterns seen with some anaesthetic agents (Biot’s, apneustic with ketamine) may also be confusing as deep or rapid ventilations may occur. Short periods of apnoea are not unusual especially following induction of anaesthesia. Overdose of anaesthetics results in prolonged periods of apnoea and a Cheyne–Stokes type of abnormal respiratory pattern in which breaths gradually increase and then decrease in depth. This pattern is seen when cerebral blood flow (CBF) is decreased and the respiratory centre becomes ischaemic (Muir & Hubbell, 2009). Cardiac arrest may be associated with specific types of patients or procedures, and at specific times during a procedure. Induction, intubation, extubation, during repositioning or movement onto or off transport carts/surgery tables, and during transport to other areas of hospital are times when conditions that could quickly lead to arrest are perhaps more likely to occur and be unnoticed. Repositioning under anaesthesia, especially from lateral to dorsal or to the opposite lateral can result in sudden severe hypotension in large animals, large dogs, and heavily pregnant females of any species, and patients with distension of the gastrointestinal tract or abdominal masses. However, small patients are not immune to problems during repositioning and even a cat may arrest immediately following a change from lateral to sternal recumbency. Dislodgement or kinking of the endotracheal tube, especially in small size patients, may also develop during repositioning. Risk of an unnoticed complication is increased when monitoring of anaesthetic depth is discontinued or decreased during the process of repositioning.
Some procedures may unexpectedly lead to arrest. Dental procedures and endoscopy of the upper airway or oesophagus have inadvertently resulted in pneumothorax, pneumomediastinum, and venous air embolism (Mitchell et al., 2000; Gunew et al., 2008; Ober et al., 2006). Laceration of a vein during limb amputation in patients breathing spontaneously and loss of the injection cap from a jugular catheter in horses can also result in venous air embolism. Insertion of central venous catheters and intraventricular pacemaker leads can stimulate cardiac arrhythmias, cause haemothorax or pneumothorax, and rarely, cardiac tamponade (Domino et al., 2004; Wess et al., 2006). Injection of contrast media or local anaesthetic intravenously or into the epidural or subarachnoid space can result in bradycardia, hypotension, and respiratory and/or cardiac arrest (Iff & Moens, 2008; Mosing et al., 2008). Ophthalmic and gastrointestinal (GI) procedures involving stimulation of parasympathetic nerves via the oculo-cardiac reflex or from tension on abdominal organs respectively, can cause bradycardia and arrest. GI procedures may also cause hypotension when diseased or large organs or masses are manipulated or exteriorized. Thoracic surgery may stimulate arrhythmias and cause haemorrhage. Manipulation of adrenal, thyroid, or pancreatic masses may also result in hypotension, hypertension, or arrhythmia (Gross & Giuliano, 2007; Greene & Marks, 2007; Harvey & Schaer, 2007).
Although cardiac arrest or situations that could lead to arrest may be anticipated more often in more critical patients, healthy patients may suffer as well when attention to monitoring is less vigilant. Anaesthetic errors persistently identified that have resulted in perioperative death include lack of monitoring, inattention to monitoring, lack of experience or knowledge, failure to treat complications such as hypotension or hypoventilation correctly, inadequate supervision, lack of communication, medication errors, and fatigue (Arbous et al., 2001, 2005; Johnston et al., 2002; Brodbelt, 2009). Medication errors such as overdose, incorrect choice of drug, improper route of administration, selection of the wrong syringe or vial, and incorrect labelling have resulted in mortality and severe morbidity (Abeysekera et al., 2005).
Risk of mortality associated with anaesthetic monitoring has been evaluated in many studies (Table 22.2). The presence of a technician to monitor the patient, the presence of experienced staff, and the use of full time staff have been associated with decreased risk of anaesthetic mortality in veterinary and human medicine. Use of documented equipment checklists, monitoring the pulse, and the use of pulse oximetry have also been associated with decreased risk of anaesthetic death. Lack of appropriate monitoring of patients during maintenance and recovery has been associated with increased anaesthetic mortality in humans. Veterinary studies have recently identified the recovery period as a factor with the deaths of 50% of dogs, and >60% of cats and rabbits, occurring postanaesthesia, especially in the first 3 hours postoperatively. These findings differ from earlier studies, suggesting that while intraoperative monitoring has improved, it should be continued into the postoperative period (Arbous et al., 2001, 2005; Brodbelt et al., 2008; Brodbelt 2009).
Table 22.2
Risk factors for perioperative mortality in horses, small animals, and humans
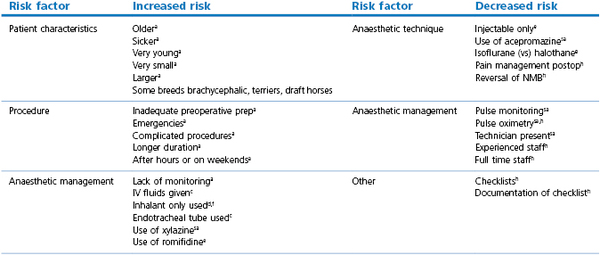
a all species;
c cats;
d dogs;
e equine;
f foals;
h human;
sa small animals.
Arbous et al., 2001, 2005; Johnston et al., 2002; Brodbelt, 2009.
Older patients, sicker patients, emergency procedures, procedures of long duration, more complex procedures, and timing of procedures after hours and on weekends have also been consistently found to be associated with greater perioperative mortality in small animals, horses, and humans (Tiret et al., 1986; Gaynor et al., 1999; Johnston et al., 2002; Arbous et al., 2005; Lienhart et al., 2006; Brodbelt, 2009).
Consistent and thorough monitoring in all patients and anticipation of potential problems in patients and situations with higher risk throughout the anaesthetic episode and recovery period will minimize the incidence of CPA and potentially improve outcome if an arrest occurs.
Cardiopulmonary cerebral resuscitation protocol
Once the heart has stopped, it cannot be actively restarted. Since the cardiac pacemaker has inherent automaticity, resuscitation efforts focus on therapies to improve coronary perfusion and eliminate any specific causes of arrest so that the cardiac pacemaker can restart on its own. Successful outcome is dependent on the prior status of the myocardium, the amount of damage that has occurred to both the heart and the brain during the arrest, and the quality of resuscitation efforts. Cerebral resuscitation is now specifically considered in the protocol as neurological status is considered to be a more important outcome parameter than return of spontaneous or sustained circulation (ROSC).
Resuscitation protocols for all patients follow the universal ‘A-B-C-D’ algorithm that represents the major components of CPCR – the airway, breathing, circulation, and drugs. Guidelines also divide the components of this algorithm into two major categories – Basic Cardiac Life Support (BCLS) and Advanced Cardiac Life Support (ACLS), even though components of both may be used simultaneously. BCLS includes the initial steps of resuscitation, the ‘A-B-C’ portion of the algorithm, where the airway, breathing, and circulation are addressed. The patient is identified as in respiratory and/or cardiac arrest, mouth to mouth or nose ventilation may be given, and cardiac compressions are initiated. ILCOR currently emphasizes the circulation portion of the algorithm more heavily because of the high incidence of sudden cardiac arrest from coronary artery disease in humans, and is now using ‘C-A-B’ to describe initial resuscitation events. Cardiac compressions and defibrillation are considered more important to outcome than medical therapy (ICCOR, 2010). ACLS includes invasive airway control, cardiac rhythm identification, medical therapy, and electrical defibrillation or conversion. Medications used for ACLS include those for treatment of a primary cause, those which improve myocardial perfusion to enable the heart to restart, and those for treatment of intra- and post-resuscitation events or conditions. The CAB algorithm may not be as applicable to veterinary anaesthesia since coronary artery disease is not seen. Distinction between BCLS and ACLS may be primarily theoretical for veterinary medicine since most resuscitation events occur in hospital where access to medical therapy and defibrillation is available. However, pet owners are becoming increasingly educated in all aspects of their pets’ health. Anecdotal reports of owner administered BCLS exist and are only likely to become more common.
Once an emergency situation has been identified, intervention must be swift, as the duration of arrest prior to both chest compressions and defibrillation is inversely related to the success of the return of spontaneous and/or sustained circulation (ROSC) and survival (Fecho et al., 2009). Although CPCR is performed by available personnel, an ideal team includes experienced individuals responsible for the following duties:
1.A leader to direct resuscitation efforts and evaluate response
2.Cardiac compressions – more than one individual to take turns
3.Ventilation of the patient, including placement of an endotracheal tube
4.Draw up and administer drugs
5.Monitors – electrocardiograph, Doppler on limb and/or cornea, capnography, oximetry
6.Vascular access – venous and arterial catheters
7.Recorder – events, medications, and time
For both ethical and economic reasons, the decision to perform resuscitation should be based on the patient’s overall prognosis in regard to the primary disease present, the suspected cause of the arrest, and the wishes of the owner. The financial and emotional costs of resuscitation and post-arrest care can be extreme. Healthy anaesthetized patients that develop a treatable arrhythmia might be easily resuscitated, whereas a dog with severe damage from a gastric volvulus, or a septic foal with uroperitoneum may not be as good a candidate for resuscitation. Cases where arrest is witnessed are also more likely to be successfully resuscitated.
Basic cardiac life support ‘A-B-C’
Once an arrest or near arrest situation has been identified in an anaesthetized patient, the inhalant anaesthetic vaporizer should be turned off and sedatives or opioids should be reversed as soon as possible. A search for potentially reversible causes such as hypoxaemia, hypercarbia, hypovolaemia, hypothermia, and metabolic or electrolyte disturbances, should also be instituted as soon as possible. This can be performed while the CPCR team assembles, someone begins compressions, and others are gathering supplies and drawing up emergency drugs.
‘A & B’ – airway and breathing
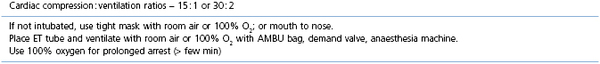
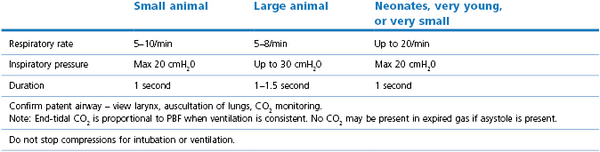
Patients under general anaesthesia are often already intubated and, in these cases, correct placement, size, and patency of the endotracheal tube should be verified and corrected if necessary. Patients who have arrested at induction, in recovery, or elsewhere should be intubated as quickly as possible. Use of a laryngoscope is recommended, when appropriate for the species, to accomplish intubation quickly and allow immediate confirmation of correct placement. Other airway devices such as a laryngeal mask airway may be used only if the operator is proficient in their use. Orotracheal intubation in many veterinary species is usually not difficult. Proper placement of the endotracheal tube should be confirmed using auscultation of lung sounds, viewing the tube entering the larynx, and capnography. Capnography is a very reliable measure as CO2 elimination is dependent on blood flow through the lungs. In patients with cardiac arrest, very low levels of end-tidal CO2 (ETCO2) are usually encountered. Negligible ETCO2 will be detected when the oesophagus has been intubated. In rare instances, no waveform may register on the capnogram even though the endotracheal tube is correctly placed, such as severe bronchospasm, severe pulmonary embolism, obstruction of the endotracheal tube, and the presence of a large leak around the endotracheal tube (Sanehi & Calder, 1999). Patients with upper airway obstruction may require intubation via a tracheostomy. A few breaths and attention to the precipitating cause (such as decreasing anaesthetic administration) may be all that is needed if the patient has suffered a primary respiratory arrest that was witnessed or very quickly identified. An AMBU bag (Fig. 22.1), demand valve (Fig. 22.2), or anaesthesia machine can be used to provide ventilation. Room air may suffice in some cases, but 100% oxygen is recommended if full cardiac arrest is present or resuscitation takes longer than a few minutes. Research and clinical investigations have shown that artificial ventilation may not be necessary for the first few minutes in some cases, the rate can be decreased from previous guidelines, and that excessive ventilation can be harmful (Aufderheide et al., 2004; Nagao, 2009). However, when arrest is associated with asphyxia, as seen with trauma, drowning, or any cause of primary respiratory arrest, especially when arrest is not witnessed and hypoxic conditions may have been present for several minutes, ventilation should be provided immediately. Cardiac compression : ventilation ratios of 15 : 1 or 30 : 2 both provided adequate oxygenation and haemodynamic profiles with minimal interruption of compressions in a dog CPR study, and are recommended when enough people are present (Hwang et al., 2008). Cardiac massage should not stop when breaths are given or intubation is performed, and compressions and ventilations should be performed simultaneously. Once intubated, ventilations should be performed in a consistent fashion at a regular rate. Hyperventilation and high peak inspiratory pressure must be avoided to avoid compromise of venous return; 10–12 breaths per minute is suggested for adult small animals, and 4–8 per minute for adult large animal species. Respiratory rate can be increased up to 20 per minute in neonates and very young animals. Inspirations should be as consistent as possible in duration and depth; approximately 1 second is recommended for most patients. Peak inspiratory pressure should be ≤20 cmH2O in small animals, and 20–30 cm H2O in adult large animals (Table 22.3) (Plunkett & McMichael, 2008; Muir & Hubbell, 2009).
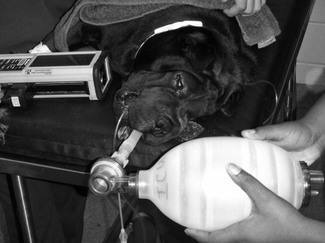
Figure 22.1 An Ambu bag being used in an anaesthetized dog. This is a manual resuscitation device originally developed by the Ambu company in Denmark over 50 years ago. It consists of a self-re-inflating bag and a non-rebreathing valve that can be used with a facemask or directly connected to an endotracheal tube. Room air is most commonly used but a line can be attached for oxygen supplementation.
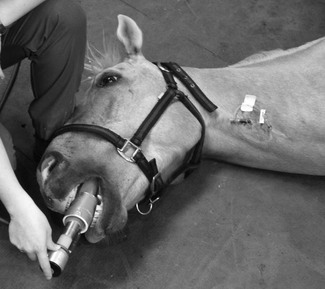
Figure 22.2 A demand valve being used in an anaesthetized horse. This is a manual resuscitation device that can be used in small or large animals to control or assist ventilation. It has a one-way valve connected via a pressure hose directly to an oxygen source. Flow of 100% oxygen is triggered by the negative pressure created by inspiration or when a button on the device is pushed. Flow stops when the button is released or the patient pauses for expiration and the negative pressure disappears. Expired gas is passively routed to the environment.
Doxapram increases ventilation by direct stimulation of the carotid chemoreceptors augmented by non-selective stimulation of central nervous system (CNS) neurons. It has been used to stimulate respiration and hasten recovery in sedated and anaesthetized small animals and horses. It may be useful if other methods to achieve breathing are not available, however, its effect is temporary. If the primary cause of respiratory arrest has not been corrected, apnoea or respiratory insufficiency will reoccur. Side effects of this drug can be significant and include cardiac arrhythmias, muscle fasciculation, seizures, increased oxygen requirement, and decreased CBF caused by the non-selective stimulation of the brain which results in increased sympathetic outflow. It is not recommended when intubation is possible and ventilation can be controlled.
Use of stimulation at the Jen Chung acupuncture point VG 26, at the nasal philtrum, is reported to stimulate respiration (Janssens et al., 1979). Although this is a simple and quick therapy, several minutes of stimulation may be required to see results, the technique has not been documented by experimental studies, and cardiac arrest occurred in two cats following nasal stimulation (K. Clarke, personal communication). Use of this technique should not preclude efforts to identify the primary cause of respiratory arrest and initiate CPCR. It is not recommended by this author.
‘C’ – circulation – cardiac compressions
In anaesthetized patients, treatment of a precipitating cause should be immediately instituted. Compressions should not be delayed or interrupted to await the outcome of any treatment. The quality of cardiac compressions has been shown to be associated with improved survival, so careful and consistent technique is important (Yu et al., 2002; Ristagno et al., 2007).
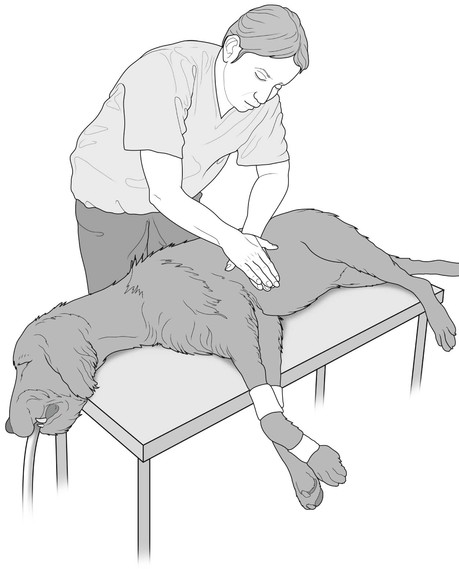
The arrested patient should be placed in right lateral recumbency on a firm surface. The individual performing compressions should be positioned above the patient to optimize compression and recoil distance. In animals 10–150 kg, the person’s hands should be placed one on top of the other at the widest part of the chest (Fig. 22.3). Compressions should be performed as consistently as possible and should depress the chest diameter at least 30–40%. The duration of the components of each compression cycle should be approximately equal in duration, i.e. 50 : 50 active compression : passive recoil. Complete recoil of the chest should be allowed between compressions to allow for maximal cardiac filling. In dogs and cats, rate of compressions should be 100–120 per minute. Compressions should continue with absolutely as few interruptions as possible. When unavoidable, interruptions should last <10 seconds. Compressions should continue following electrical shock or medical therapy for at least 2 minutes as continued perfusion of the coronary circulation is necessary for therapy to be effective. Even good compression technique produces only 20–25% of normal cardiac output in dogs. Coronary perfusion drops dramatically with cessation of compressions, and it then takes a few minutes for maximum coronary perfusion to return when compressions are resumed. Performance of external compressions is tiring and multiple rescuers should take turns at approximately 2-minute intervals (Box 22.1).