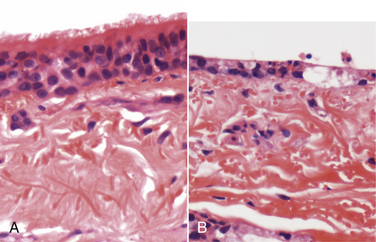
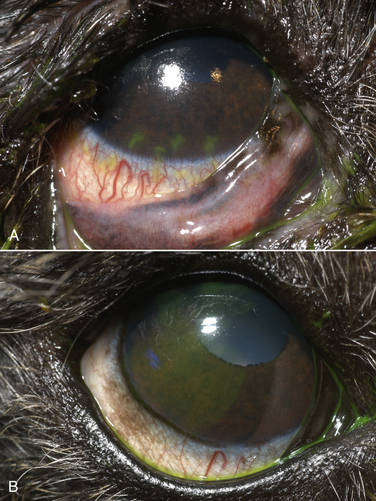
Chapter 17 Canine viral respiratory disease is a widespread problem where large numbers of dogs are housed indoors together, such as in shelters, commercial dog colonies, and breeding facilities. The longer dogs are housed in a shelter situation, the greater the risk that respiratory illness will occur.3 In shelter environments, transmissible respiratory disease (or canine infectious respiratory disease complex [CIRDC], previously referred to as “kennel cough” or canine infectious tracheobronchitis) delays the placement of dogs in homes and can result in unmanageable costs related to treatment, quarantine, and isolation. Transmissible respiratory disease occasionally occurs in owned dogs after a period of contact with large numbers of other dogs at dog parks, dog sporting events such as fly ball, or dog behavior classes. It can also occur after dogs (or dog owners) visit veterinary hospitals, boarding facilities, or pet daycare centers. With the widespread clinical application of molecular diagnostic assays, it is increasingly apparent that the number of viruses that can infect the canine respiratory tract is much larger than previously thought. This has led to exciting new discoveries in the field of canine infectious respiratory disease, and our knowledge of the pathogens involved continues to expand. Co-infections with multiple viruses and bacteria such as Mycoplasma spp., Bordetella bronchiseptica, and Streptococcus equi subspecies zooepidemicus are common and contribute to an increased severity of disease.4–6 Viruses believed to play a role in canine respiratory disease include canine herpesvirus-1 (CHV-1), canine adenovirus-2 (CAV-2), canine distemper virus (CDV), canine parainfluenza virus (CPiV), canine pneumovirus, canine respiratory coronavirus (CRCoV), and canine influenza virus (CIV) (Table 17-1). CAV-1 may also play a role when vaccination is not performed or when it is performed improperly.7 Reoviruses may also play a role.8 CDV, CHV-1, and CAV are discussed in more detail in Chapters 15, 16, and 18, respectively. The major bacterial causes of canine transmissible respiratory disease are covered in Part II, Section 2 of this book (Chapters 34, 38, and 40). Although most of the canine respiratory viruses have a worldwide distribution, their relative prevalence varies from year to year and between geographic locations. Even within a state or city, predominant pathogens may differ from one shelter and boarding kennel to another. TABLE 17-1 Potential Causes of Transmissible Respiratory Disease in Dogs ∗Incubation and shedding periods are approximate and may differ when co-infections or immunosuppression operate. Adenoviruses are icosahedral DNA viruses that infect a variety of animal species. CAV-2 is found worldwide and primarily infects the respiratory tract of dogs. Rarely, it has been implicated as a cause of enteritis in dogs, and it was found in the brains of puppies with neurologic signs.9 The virus replicates in nonciliated bronchiolar epithelial cells; epithelial cells of the nasal mucosa, pharynx, and tonsillar crypts; mucous cells in the trachea and bronchi; and type 2 alveolar epithelial cells. CAV-2 can also be isolated from retropharyngeal and bronchial lymph nodes as well as epithelial cells of the intestinal tract. Shedding typically ceases 1 to 2 weeks after initial infection. The extent to which CHV-1 plays a role in respiratory disease in dogs has been debated.10 Experimental infections of dogs can result in rhinitis or signs of tracheobronchitis, and intraocular infection results in conjunctivitis and keratitis.11–14 There is evidence of widespread exposure to CHV-1 in dogs worldwide,10 and a substantial proportion of the dog population may be latently infected. Like other herpesviruses, CHV-1 becomes latent in neurologic tissues, with precipitation of virus shedding by stress. Consistent with the time delay required for reactivation after stress, CHV-1 was most frequently detected 3 to 4 weeks after dogs were introduced to a rehoming center, whereas CRCoV and CPiV were most frequently detected in the first and second week. Dogs infected with CHV-1 were more likely to have severe respiratory disease, although severe respiratory disease itself might predispose dogs to the shedding of CHV-1.10 Infection with CHV-1 was reported in association with fatal hepatic necrosis in an adult dog that lacked any evidence of immunosuppression.15 Refer to Chapter 16 for information on CHV-1 infections in neonates. Influenza viruses are enveloped viruses with segmented single-stranded RNA genomes that belong to the family Orthomyxoviridae. Influenza viruses that cause disease in domestic animals belong to the genus Influenzavirus A, whereas influenza B and influenza C viruses primarily circulate among humans. Influenza A viruses are classified based on the genetic composition of their hemagglutinin (H) and neuraminidase (N) genes. To date, 16 H types and 9 N types have been identified, each of which are antigenically distinct.16 The names of influenza viruses are specified as follows: influenza genus (A, B, or C)/host/geographic origin/strain number/year of isolation and, in parentheses, H and N type—for example, A/canine/Florida/43/2004 (H3N8).17 Extensive genomic rearrangements that occur within influenza A viruses allow for occasional cross-species transmission among birds and mammals. These rearrangements occur when two different viruses simultaneously infect a host, with subsequent genetic reassortment. Occasionally, cross-species transmission occurs without alteration of the viral genome. In the United States, CIV emerged in racing greyhounds in Florida in 2003 and 2004,17 where it caused hemorrhagic pneumonia and a high mortality. However, serologic evidence of infection in the greyhound dog population dates back to 1999.18 Infections spread slowly and were subsequently reported in racing greyhounds and non-greyhounds in at least 38 U.S. states. Outbreaks have continued to occur in shelter situations for nearly a decade after the virus was discovered. The virus that currently circulates in the United States is an H3N8 virus that closely resembles an equine influenza virus, which suggested that an interspecies jump occurred without genetic reassortment.17 Instead, accumulation of point mutations with minor amino acid changes occurred, with sustained transmission among dogs. The most significant outbreaks of disease due to CIV have occurred in Florida, New England, Colorado, Wyoming, and Texas. In many other states, sustained transmission of the virus from one dog to another has not occurred. The most significant risk factor for infection has been indoor housing.19 Virtually all cases to date have involved dogs in kennels, animal shelters, or dog daycare facilities. Dogs of all ages and breeds are susceptible, but to date severe hemorrhagic pneumonia has occurred only in greyhounds.20 The virus is shed for up to 7 to 10 days. CIV has retained the ability to infect horses, but horses develop only mild disease or no clinical signs.20 Avian-lineage H3N2 CIV emerged in South Korean dogs in 2007, and a similar virus was subsequently isolated from dogs in China.21,22 Experimentally, cats are also susceptible to infection by this virus.23 Korean isolates were associated with epidemics of respiratory disease in kennels within veterinary clinics.22 A novel H3N1 virus was also detected in South Korean dogs that lacked signs of respiratory disease.24 A novel H5N2 influenza virus was recently detected in a dog with respiratory disease in China.25 Dogs are susceptible to infection with human influenza virus H1N126,27 and avian H5N1,28 but sustained transmission of these viruses in the dog population has not been reported. Limited infection of dogs with equine H3N8 viruses was detected in hounds in England29 and during an equine influenza outbreak in Australia.30 In England, disease was so severe that several hounds had to be euthanized, and subacute bronchointerstitial pneumonia was detected at necropsy.29 The Australian dogs developed inappetence, lethargy, nasal discharge, and a cough that persisted for several weeks, but dog-to-dog transmission was not identified. Experimental transmission of H3N8 influenza virus from horses to dogs was documented in Japan, but infected dogs did not show signs of illness.31 CDV, CPiV, and canine pneumovirus belong to the family Paramyxoviridae, which are enveloped RNA viruses. CPiV belongs to the genus Rubulavirus. Although previously referred to as canine parainfluenza virus-2, it is probably the same virus as simian virus 5, which was originally isolated from monkey cell cultures. It has been proposed that it be renamed parainfluenza virus 5.32 Unlike CDV, the outer envelope possesses not only hemagglutinin but also neuraminidase activity (HN attachment glycoprotein). The virus infects dogs worldwide, replicates in epithelial cells of the upper respiratory tract, and often causes no signs or mild respiratory illness. Respiratory disease may be more severe when co-infections with other pathogens such as B. bronchiseptica are present.33 Viremia seems to be uncommon, but occasionally CPiV has been isolated from the liver, spleen, and kidneys. A strain of CPiV was also isolated from a dog with neurologic signs.34 There is some evidence that cats may be infected with CPiV or a closely related virus.35 Virus is shed for up to 10 days after infection. Canine pneumovirus belongs to the genus Pneumovirus. It was first isolated from dogs with acute respiratory disease in shelters in the United States in 2010.2 The virus is most closely related to a murine pneumovirus and in fact can replicate in mice and cause severe inflammatory pathology.36 Reoviruses (family Reoviridae, genus Orthoreovirus) are non-enveloped viruses with a segmented, double-stranded RNA genome. Mammalian reoviruses infect a variety of host species and have a worldwide distribution. The prefix reo- stands for respiratory enteric orphan virus, which highlights the tropism of reoviruses for cells of the respiratory and gastrointestinal tract, and their uncommon association with disease. Despite serologic evidence of widespread exposure to reoviruses in dogs, they have been found only rarely in dogs with respiratory disease8,37 and dogs with enteritis.38 There are three mammalian reovirus (MRV) serotypes, and all three have been detected in dogs. The role of reoviruses in disease causation in dogs is unclear, because disease has not been reproducible experimentally. It has been speculated that reoviruses might act synergistically with other respiratory pathogens to cause disease.10 Coronaviruses are enveloped RNA viruses that possess large, club-shaped spikes on their outer surface, also known as peplomers (see Figure 14-1, B). CRCoV is a relatively newly identified cause of contagious respiratory disease in dogs. The virus is a group 2a coronavirus (family Coronaviridae, genus Coronavirus) and is distinct from canine enteric coronavirus, a group 1a coronavirus (Box 17-1).39 Minimal serologic cross-reactivity exists between these two viruses. Group 2a coronaviruses possess a gene that encodes hemagglutinin esterase, an outer membrane protein glycoprotein. This gene is absent in group 1 and 3 coronaviruses. CRCoV is most closely related to a bovine coronavirus but also resembles human coronavirus OC43.40 CRCoV was first reported in 2003, in a group of dogs with respiratory disease in a rehoming facility in England that had been vaccinated against CAV-2, CDV, and CPiV.6,40 Some of the dogs were co-infected with CPiV and CHV-1.6 CRCoV spread rapidly and primarily was detected in the first week that dogs were introduced to the kennel, after which time CPiV and CHV-1 were detected. Alone, CRCoV causes subclinical infections or mild respiratory disease, but like human respiratory coronaviruses, it can cause reversible damage to, or loss of, the cilia on respiratory epithelial cells (Figure 17-1). As a result, infected dogs are predisposed to secondary infections. Serologic evidence of exposure to CRCoV is widespread in dogs from North America, Great Britain and continental Europe, Japan, Korea, and New Zealand,10,41–43 and the virus has been detected widely using PCR-based methods in dogs with respiratory disease from many of these countries. To date, there is no evidence that cats can be infected with CRCoV. FIGURE 17-1 Effect of canine respiratory coronavirus infection on ciliated respiratory epithelial cells.A, Respiratory epithelium immediately before inoculation with CRCoV. B, The same cells 48 hours after inoculation with CRCoV. There is marked loss of cilia, thinning of the epithelium with individual cell necrosis, and sloughed necrotic cells on the surface. There are also occasional large vacuoles within the epithelium, which likely represent foci of cell loss secondary to viral replication. (Courtesy Dr. Simon Priestnall and Professor Joe Brownlie, Royal Veterinary College, UK.) Infection with pancytotropic strains of canine enteric coronavirus has recently been associated with severe systemic disease in puppies from Europe. Affected pups have fever, mucoid to hemorrhagic enteritis, lymphopenia, neurologic signs such as seizures, and bronchopneumonia.44,45 Co-infections with CPV-2 were present in some puppies, but the disease has been reproduced by experimental infection by canine enteric coronavirus alone. Death occurred in some puppies, but others developed transient gastrointestinal signs and recovered.46 The incubation period for viral respiratory disease is generally less than 2 weeks and can be as short as 2 to 3 days. Influenza virus infections, in particular, have been associated with very short incubation periods.20 Transmission occurs by aerosol, but direct contact between dogs and fomite transmission (hands, clothing, contaminated food and water bowls, common hallways and exercise areas) are important routes in population-dense environments. Cells of the larynx, trachea, bronchi, and sometimes the nasal mucosa, bronchioles, and alveoli are infected, and although viral shedding patterns differ between pathogens, shedding may occur before the onset of clinical signs. The duration of shedding for CIV and CPiV is very short, typically a few days, and in some dogs, shedding has ceased by the time clinical signs are most apparent. In contrast, CDV can be shed for weeks (see Chapter 15). Infection with respiratory viruses may be associated with no signs, or complicated pneumonia and death can occur. In general, morbidity is high, but mortality is low. Moderate to severe signs may be more likely to occur in very young puppies, genetically susceptible animals, and when stress and co-infections with multiple viral and bacterial pathogens are present. Infection with CDV is especially effective at predisposing dogs to other respiratory viral infections, because of its immunosuppressive properties, but other viruses that damage ciliated epithelial cells, such as CRCoV and CPiV, also predispose to co-infections. Clinical signs of acute respiratory disease include mild fever, a paroxysmal harsh or “honking” cough, serous nasal discharge, and sometimes sneezing, but usually otherwise affected dogs are often alert, active, and appetent. The cough may be followed by gagging or retching, which may be followed by the production of frothy mucus. An altered bark or stridor may occur in dogs that develop laryngitis, tonsillitis, and/or pharyngitis. CAV-2 and CHV-1 may cause conjunctivitis.47 In addition, CHV-1 has been associated with ulcerative and nonulcerative keratitis, which may be precipitated by immunosuppression (Figure 17-2).48–50 Infections with CIV or CDV, or infections with multiple respiratory pathogens, may be more likely to produce systemic signs of fever and lethargy. Dogs that develop secondary bacterial infections may show fever, inappetence, lethargy, a mucopurulent nasal discharge, tachypnea, and a moist, productive cough. FIGURE 17-2 A, Right eye of a 10-year-old male neutered miniature schnauzer infected with bilateral conjunctivitis and dendritic ulceration associated with canine herpesvirus infection. Fluorescein uptake can be seen in a branching pattern at the limbus of the right eye. B, The same dog 1 month later. Note diffuse stain uptake over at least 25% of the corneal surface. (Courtesy University of California, Davis Veterinary Ophthalmology Service.) On physical examination, dogs with uncomplicated transmissible respiratory disease are typically bright, alert, and active with a paroxysmal cough. The cough is often easily elicited on tracheal palpation. Conjunctivitis and serous ocular and/or nasal discharge may be present, and the tonsils may be enlarged and hyperemic. Dogs with secondary bacterial infections may be pyrexic, lethargic, and tachypneic with increased respiratory effort and increased lung sounds on thoracic auscultation. A mucopurulent nasal discharge may also be present. Ulcerative or nonulcerative keratitis may be present in dogs infected with CHV-1. Most dogs experience self-limiting disease. Attempts to obtain an etiologic diagnosis should be made when disease persists for longer than 7 to 10 days or is complicated by bacterial pneumonia, with lethargy and inappetence. When outbreaks occur in shelters or the pattern of endemic respiratory disease changes, attempts to make an etiologic diagnosis are also indicated and encouraged (Table 17-2). Collection of multiple specimen types from several dogs with and without clinical signs in an outbreak situation can facilitate diagnosis and allow interpretation of the significance of positive test results. Organism detection methods, such as PCR assays, are likely to be of highest yield early in the course of illness (e.g., the first 1 to 3 days) or in exposed dogs that have not yet developed clinical signs. Use of a combination of serology and organism detection methods also facilitates diagnosis. In shelter situations or outbreaks where severe disease occurs, necropsies can provide valuable information and should be performed by a veterinary pathologist as soon as possible after death or euthanasia. Tissues should be submitted for histopathology (in formalin), bacterial and virus cultures (fresh tissue), and PCR assay for respiratory viruses and bacteria (fresh or frozen tissue; see Chapter 5).
Canine Viral Respiratory Infections
Etiology and Epidemiology
Canine Adenovirus-2
Canine Herpesvirus-1
Canine Influenza Virus
Canine Parainfluenza Viruses
Canine Reoviruses
Canine Respiratory Coronavirus
Clinical Features
Physical Examination Findings
Diagnosis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree