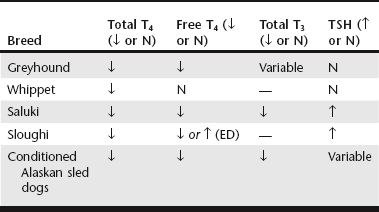
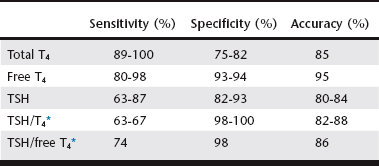
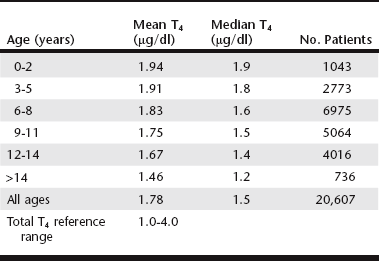
Chapter 42 Any breed can develop hypothyroidism; however, some breeds, such as the golden retriever and the Doberman pinscher, have been reported to be at higher risk. Thyroiditis is clearly heritable in the beagle and the borzoi, and many other common breeds, such as the golden retriever, Great Dane, Irish setter, Doberman pinscher, and Old English sheepdog, have a high prevalence of ATAs. The rate of clinical progression of thyroiditis also varies among breeds. In beagles, the prevalence of thyroiditis can reach 40%, but if thyroiditis progresses to hypothyroidism, it occurs in middle age or later (Scott-Moncrieff et al, 2006). In other breeds, such as golden retrievers, thyroiditis appears to progress more rapidly, with some dogs diagnosed at 2 years of age. Middle-aged dogs generally are at increased risk of hypothyroidism. In one study, mean age at diagnosis was 7 years (range, 0.5 to 15 years). Dermatologic findings include dry scaly skin, changes in hair coat quality or color, symmetrical alopecia, seborrhea, and superficial pyoderma. Hyperkeratosis, hyperpigmentation, comedo formation, hypertrichosis, ceruminous otitis, poor wound healing, increased bruising, and myxedema also may occur. Alopecia is usually bilaterally symmetrical (see Chapter 39) and is first evident in areas of wear, whereas the head and extremities tend to be spared. The hair may be brittle and easily epilated, and loss of undercoat or primary guard hairs may result in a coarse appearance or a puppy-like hair coat. Fading of coat color may occur, and failure of hair regrowth after clipping is common. Hypothyroid dogs are also predisposed to recurrent bacterial infections of the skin. Reproductive dysfunction has been attributed to hypothyroidism. There is little evidence for male reproductive dysfunction associated with hypothyroidism; however, bitches with radioactive iodine (131I)–induced hypothyroidism have reduced fertility, prolongation of parturition, and higher periparturient mortality compared with euthyroid bitches (Panciera et al, 2007). Both the peripheral nervous system and the central nervous system may be affected by hypothyroidism. Subclinical myopathy is well documented in hypothyroid dogs (Rossmeisl, 2010). Peripheral nerve dysfunction also has been described in association with hypothyroidism. Affected dogs have exercise intolerance, generalized weakness, ataxia, tetraparesis or paralysis, deficits of conscious proprioception, and decreased spinal reflexes. Some affected dogs have multifocal dysfunction of cranial nerves (facial, trigeminal, vestibulocochlear). Clinical signs resolve with levothyroxine sodium (l-thyroxine) supplementation. Rarely, central neurologic dysfunction (seizures, central vestibular dysfunction, disorientation, and mentation changes) is observed as a result of cerebral or cerebellar infarction, atherosclerosis, myxedema, coma, and blood viscosity changes secondary to hyperlipidemia. Overall, hypothyroidism is a rare cause of seizure disorders in dogs (Higgins et al, 2006). Although laryngeal paralysis and megaesophagus have been reported in association with hypothyroidism, treatment of hypothyroidism does not consistently result in resolution of clinical signs, and a causal relationship has not been established. Behavioral abnormalities that have been attributed to canine hypothyroidism include aggression and cognitive dysfunction; however, evidence for a causal association between behavioral problems and hypothyroidism is lacking. Congenital hypothyroidism results in mental retardation and stunted, disproportionate growth caused by epiphyseal dysgenesis and delayed skeletal maturation. Affected dogs are mentally dull and have large broad heads, short thick necks, short limbs, macroglossia, hypothermia, delayed dental eruption, ataxia, and abdominal distention. A palpable goiter may be present, depending on the cause of the congenital defect. Other clinical signs include gait abnormalities, stenotic ear canals, sealed eyelids, and constipation. Congenital hypothyroidism with goiter (CHG) caused by a nonsense mutation in the thyroid peroxidase gene has been recognized in toy fox terriers and rat terriers (Fyfe et al, 2003; Dodgson et al, 2012). A different missense mutation of the thyroid peroxidase gene causes CHG in Tenterfield terriers. Both defects are autosomal-recessive traits, and a deoxyribonucleic acid test that detects carriers of the defects is available through the laboratory of comparative medical genetics at Michigan State University. T4 is the major secretory product of the thyroid gland, whereas most serum T3 is derived from the extrathyroidal deiodination of T4. Both T3 and T4 are highly protein bound to serum carrier proteins. Only unbound (free) hormone penetrates cell membranes, binds to receptors, and has biologic activity. Protein-bound hormone acts as a reservoir and buffer to maintain a steady concentration of free hormone in the plasma despite rapid alterations in release and metabolism of T3 and T4 and changes in plasma protein concentrations. Free T4 is monodeiodinated within cells to T3, which binds to receptors and induces the cellular effects of thyroid hormone. Basal thyroid hormone measurements used in the diagnosis of canine hypothyroidism are shown in Table 42-1. TABLE 42-1 Diagnostic Tests for Hypothyroidism in Dogs T4, Thyroxine; TSH, thyroid-stimulating hormone. *A dog was considered to have hypothyroidism only if the T4 or free T4 was decreased and the TSH was increased. Factors such as age, breed, drug therapy, and concurrent illness influence thyroid hormone concentrations without altering metabolically active free thyroid hormone concentrations. Not only do thyroid hormone concentrations in healthy dogs commonly fluctuate out of the reference range, but also numerous breeds have been identified as having thyroid hormone concentrations different than established laboratory reference ranges (Table 42-2). There is also a significant decline in thyroid hormone concentrations with age in dogs (Table 42-3), and many drugs have a marked influence on the thyroid axis (Table 42-4) (Daminet et al, 2003). Dogs treated with phenobarbital that have clinical signs of hypothyroidism are particularly challenging to evaluate for hypothyroidism because phenobarbital treatment causes both a decrease in thyroid hormone concentrations and sometimes an increase in TSH. Options include reevaluation after withdrawal of phenobarbital (and, if necessary, transition to an alternative drug) or consideration of a therapeutic trial (see later). TABLE 42-3 Mean and Median Serum Total Thyroxine Concentrations of Samples Submitted to a Reference Laboratory for Dogs of Different Ages* *Patients with age listed as zero were excluded. Data provided by IDEXX Laboratories, Inc., Westbrook, ME.
Canine Hypothyroidism
Signalment
Clinical Signs
Diagnosis
Basal Thyroid Hormone Concentrations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine Hypothyroidism
Only gold members can continue reading. Log In or Register to continue