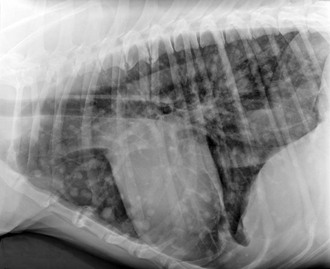
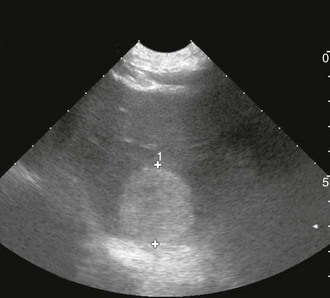
Chapter 88 The definitive etiology of canine HSA remains uncertain, although the strong breed association suggests that heritable factors are present to explain this genetic predisposition, and in fact gene expression phenotypes were shown to vary in HSA cells from different breeds in a recent study (Tamburini et al, 2009). Long-term ultraviolet light exposure is a known risk factor for superficial (dermal) HSA in lightly pigmented short-haired breeds of dogs and appears also to be a risk factor for HSA in the conjunctival location. With the advent of platforms enabling evaluation of global gene expression, it soon may be possible to identify which genes and pathways are dysregulated in HSA. One recent study noted differences between HSA cells and nonmalignant endothelial cells in regard to increased expression of genes involved in inflammation, angiogenesis, adhesion, invasion, metabolism, cell cycle, signaling, and patterning. Importantly, this “signature” not only reflected a cancer-associated angiogenic phenotype but could distinguish HSA from other nonendothelial, angiogenic bone marrow–derived tumors such as lymphoma, leukemia, and osteosarcoma (Tamburini et al, 2009, 2010). Canine HSA can develop anywhere in the body. The four most common primary sites are the spleen, heart (right atrium or auricle), skin or subcutaneous tissues, and liver. Other reported primary sites include kidney, muscle, bone, oral cavity, bladder, and lung. Metastatic dissemination and local infiltration occur early in disease, either hematogenously or via local seeding following tumor rupture. The lungs, liver, and omentum are the most frequent sites of dissemination, but HSA also is recognized as the most common sarcoma to metastasize to the central nervous system (CNS). Certain primary tumor locations may predict a better prognosis, and dermal, conjunctival, and possibly subcutaneous HSA tend to have a lower metastatic rate than visceral locations. Studies have demonstrated that higher clinical stage (Box 88-1) results in earlier metastasis and shorter survival time for splenic and cutaneous HSA. When advanced metastatic disease is present, it is sometimes impossible to identify the primary site, and the prognosis is unsurprisingly poor to grave, even though occasional responses to therapy have been observed. As a result, complete clinical staging ideally should include three-view high-detail thoracic radiography, abdominal ultrasonography, and echocardiography, in addition to standard blood studies. Although the typical appearance of measurable pulmonary metastatic disease is that of a coalescing miliary pattern, nodular or generalized miliary interstitial patterns also can be observed on occasion (Figure 88-1). Abdominal ultrasonography is a fairly sensitive routine imaging technique permitting the detection of splenic or hepatic lesions (Figure 88-2) and occasionally of omental nodules as well, and in most cases will identify free abdominal fluid when present. Early experience of the use of contrast-enhanced ultrasonography suggest that this technique may become more common in the veterinary setting; when the echogenicity of hepatic nodules is evaluated during arterial and parenchymal phases of contrast enhancement, malignant tumors can be differentiated from benign nodules with high rate of accuracy. Echocardiography remains the method of choice to identify right atrial masses, and these are better observed when at least some amount of pericardial effusion is present. Although urinalysis and serum biochemical studies rarely help in the diagnosis of HSA, the complete blood cell count typically shows changes that may suggest a microangiopathic process, including regenerative anemia, thrombocytopenia, and fragmented red blood cells (schistocytes). In addition, when the spleen is affected severely, a deficient reticuloendothelial system allows higher numbers of metarubricytes (nucleated red blood cells) to be observed in the circulation and on manual blood smears. Figure 88-1 Left lateral thoracic digital radiograph of a 6-year-old Irish wolfhound, demonstrating a nodular pattern of pulmonary metastasis from intramuscular hemangiosarcoma. Figure 88-2 Abdominal ultrasonographic image of a 9-year-old Labrador retriever demonstrating a heterogeneous liver nodule, later confirmed histopathologically to be a primary liver hemangiosarcoma.
Canine Hemangiosarcoma
Causes
Biologic Behavior and Prognosis
Diagnosis and Staging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


