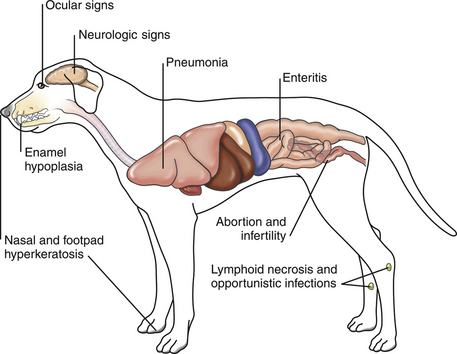
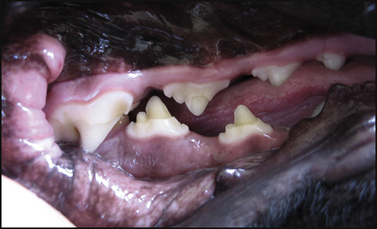
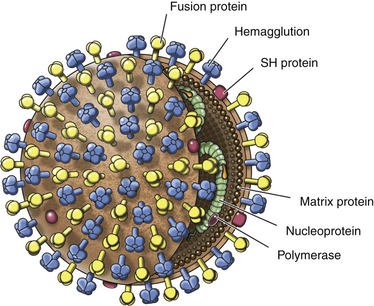
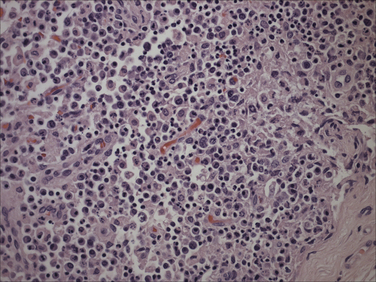
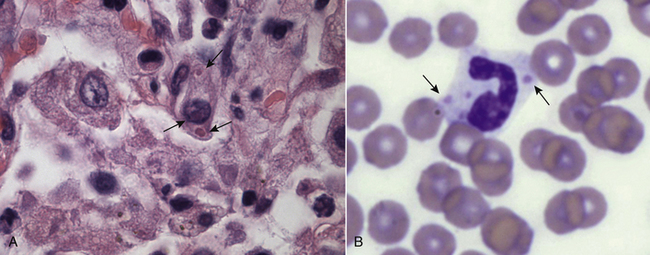
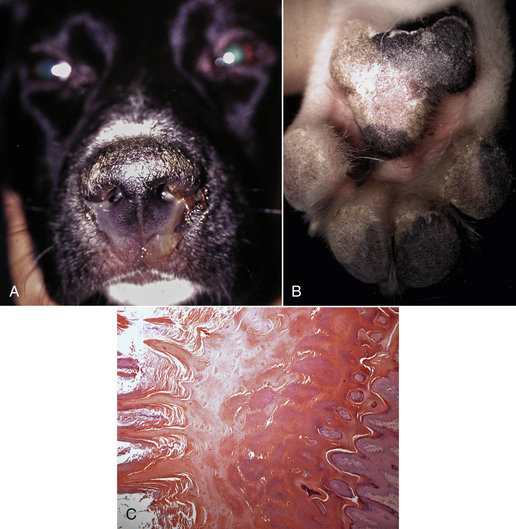
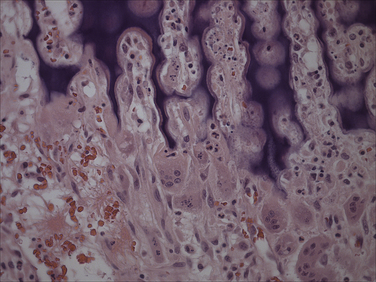
Chapter 15 Canine distemper is an important disease of domestic dogs and wild animals worldwide. It is caused by canine distemper virus (CDV), an enveloped, pleomorphic RNA virus that belongs to the genus Morbillivirus (family Paramyxoviridae). CDV is closely related to human measles virus and rinderpest virus and has been used as a model to study the pathogenesis of measles virus infections. The outer envelope of CDV contains hemagglutinin (H) and fusion (F) proteins, which are important in cellular attachment and entry (Figure 15-1). Infection of dogs can lead to a severe, multisystemic disease that primarily affects the gastrointestinal, respiratory, and neurologic systems (Figure 15-2). FIGURE 15-1 Structure of CDV. The outer envelope contains hemagglutinin (H) and fusion (F) proteins, which are connected to the nucleocapsid by a matrix (M) protein, which is the most abundant protein in the virion. Worldwide, at least eight different geographic lineages (also referred to as genotypes or clades) of CDV exist based on sequence analysis of the H gene: Asia-1, Asia-2, America-1, America-2, Europe-1/South America-1, Europe-2 (European wildlife), Europe-3 (Arctic-like), and South Africa. In addition, strains recognized in Argentina, Africa, Asia, and Mexico may represent separate geographic lineages.1,2 In the United States, vaccine strains belong to the America-1 lineage, whereas most field strains belong to the America-2 lineage. Strains that belong to the Europe-2 and Europe-3 lineages have also been detected in North American dogs.3 Although vaccination has reduced the incidence of the disease, distemper still remains important where large numbers of young dogs with inadequate immunity are housed together, such as in kennels, large breeding facilities, and shelter environments. Puppies that have lost their maternal antibody are predisposed in regions where vaccination is performed, but occasionally disease occurs in older, vaccinated dogs.4–6 Distemper also occurs in regions where vaccination of dogs is not performed or is poorly timed. As an enveloped virus, CDV is susceptible in the environment (surviving less than 1 day at room temperature) and is readily inactivated by heat, drying, and disinfectants; thus, contact between infected dogs is important to maintain transmission of the virus. CDV is highly contagious and is spread through droplet nuclei and large-particle aerosol transmission. Dogs are generally exposed to CDV through contact with infected oronasal secretions, which may be shed by subclinically or clinically affected dogs. The virus initially infects monocytes within lymphoid tissue in the upper respiratory tract and tonsils and is subsequently disseminated via the lymphatics and blood to the entire reticuloendothelial system. The viral hemagglutinin binds to a molecule on the surface of host cells known as the signaling lymphocytic activation molecule (SLAM, also known in humans as CD150). SLAM is a membrane glycoprotein of the immunoglobulin superfamily. It is important for cell entry by CDV as well as other morbilliviruses and is a key molecule in the pathogenesis of disease.7 SLAM is expressed by immature thymocytes, activated lymphocytes, macrophages, and dendritic cells. Direct viral destruction of a significant proportion of the lymphocyte population, and especially CD4+ T cells, occurs within the blood, tonsils, thymus, spleen, lymph nodes, bone marrow, mucosa-associated lymphoid tissue, and hepatic Kupffer cells (Figure 15-3).8–10 Massive destruction of lymphocytes results in an initial lymphopenia and transient fever, which occurs a few days after infection. Subsequently, there is a second stage of cell-associated viremia and fever (8 to 9 days after infection), after which CDV infects cells of the respiratory, gastrointestinal tract, central nervous system (CNS), urinary tract, and skin, as well as red and white blood cells, including additional lymphoid cells. In this stage of infection, CDV infects a variety of cell lineages, including epithelial, mesenchymal, neuroendocrine, and hematopoietic cells, and forms intracytoplasmic but also intranuclear inclusions (Figure 15-4). Infection of epithelial cells by the virus is inefficient and occurs relatively late in the course of infection, through a SLAM-independent mechanism that appears to involve the nectin 4 receptor, as is true for measles virus.11 CDV is shed in all secretions and excretions from as early as day 5 after infection, before the onset of clinical signs. Shedding of virus can continue for as long as 3 to 4 months, but usually resolves after 1 to 2 weeks. FIGURE 15-3 Lymph node histopathology of a 5-month-old German shepherd mix with distemper. The dog had been adopted from a shelter and subsequently developed neurologic signs, diarrhea, and pneumonia. There is marked lymphoid necrosis. H&E stain. FIGURE 15-4 A, Eosinophilic intracytoplasmic and intranuclear viral inclusions (arrows) in the lung of the dog from Figure 15-3. Hematoxylin and eosin stain, 1000× oil magnification. B, Intracytoplasmic inclusions (arrows) in a circulating leukocyte of a 4-month-old Border collie mix with distemper. Up to 30% of infected dogs develop CNS signs. These usually occur 1 to 6 weeks after the onset of acute illness but can occur even when initial infection is subclinical. Neurologic signs are more likely to develop following infection with certain CDV strains, such as the Snyder Hill strain.12 The types of brain cells that are infected differ with the strain of CDV involved,13 but neuronal necrosis and atrophy can occur.14 Neurologic signs are often progressive and generally do not resolve, so dogs that recover often have residual neurologic deficits. “Old dog encephalitis” is a poorly characterized, progressive immune-mediated demyelinating leukoencephalomyelitis induced by CDV that occurs weeks to years after recovery from the acute infection and is difficult to diagnose. The term “old dog encephalitis” is a misnomer, because affected dogs are not necessarily old. Both CDV and measles virus have been incriminated as possible agents of multiple sclerosis because of the resemblance of demyelinating CDV encephalitis to the pathology of this disease.15 Virus has been detected within the brains of these dogs with immunohistochemistry and reverse-transcriptase PCR assays.16 Persistent infection of astrocytes in the CNS by some CDV strains has been postulated as a mechanism for this chronic manifestation of infection, with transmission from cell to cell through induction of cell-cell fusion and resultant evasion of the host immune response.17 Ocular signs of persistent CDV infection include uveitis, chorioretinitis, keratoconjunctivitis sicca (KCS), keratitis, and optic neuritis, which may be associated with blindness. KCS is thought to result from damage to the lacrimal gland by CDV.18 It may be transient or permanent, and can lead to keratitis and corneal ulceration. The virus itself can also infect the corneal epithelial and stromal cells. Enamel and dentin hypoplasia, manifested as irregularities of the teeth, is an incidental finding in recovered dogs due to infection of the stratum intermedium of developing teeth (Figure 15-5). Retention and partial eruption of teeth has also been reported as a sequela of CDV infection.19,20 Infection of the skin can lead to a cutaneous measles-like rash, although this is uncommonly recognized. Persistent infection of the footpad and nasal planum epithelium leads to hyperkeratosis in these regions (Figure 15-6). FIGURE 15-6 Nasal planum (A) and footpad (B) hyperkeratosis induced by CDV infection in a 4-month-old Border collie mix with conjunctivitis, mucopurulent nasal and ocular discharge, and myoclonus. Blepharospasm is also present. C, Histopathology of the footpad of a 1-year old German shepherd mix with distemper. Marked hyperkeratosis is evident. H&E stain, 40× magnification. Immunosuppression results from lymphopenia, necrosis of hematopoietic cells in the bone marrow, and dendritic cell malfunction. Lymphocyte apoptosis also occurs independent of viral infection of lymphocytes.21 The viral V protein allows CDV to replicate rapidly in T cells and is critical in CDV-mediated immunosuppression. This protein almost completely antagonizes IFN-α, TNF-α, Il-6, IFN-γ, and Il-2 in the acute phase of infection.10 Finally, the nucleocapsid (N) protein of morbilliviruses binds to the CD32 (Fc-γ) receptor on B cells, which results in impaired differentiation of B cells into plasma cells.22 When the virus binds this receptor on dendritic cells, impaired antigen presentation by dendritic cells results, which disrupts T-cell function. The most common secondary infections in distemper are secondary bacterial infections that contribute to bronchopneumonia. Bordetella bronchiseptica is a common copathogen. Dogs may be diagnosed with bordetellosis in the early stages of distemper, and the underlying CDV infection may be overlooked. Other opportunistic infections that have been identified in dogs with distemper include toxoplasmosis,23 salmonellosis,24 nocardiosis,25,26 and generalized demodecosis. Infection with Pneumocystis carinii was associated with CDV infection in a mink,27 and concurrent neosporosis and canine distemper was reported in a raccoon.28 Uncommonly, CDV infection has been associated with metaphyseal bone lesions in young dogs as a result of infection of osteoclasts, osteoblasts, and osteocytes.29 Infected cells undergo degeneration and necrosis, and metaphyseal osteosclerosis occurs (Figure 15-7). This may lead to pain and lameness. CDV RNA has also been detected in bone cells of dogs with hypertrophic osteodystrophy, and CDV may play a role in this disease.30 FIGURE 15-7 Severe, neutrophilic osteomyelitis with submetaphyseal osteonecrosis and extensive bony resorption in a 4-month-old intact male Labrador retriever mix with distemper. The dog was seen for lethargy, mild diarrhea, fever, and reluctance to stand; joint pain was detected on physical examination. Direct IFA on urine sediment was positive for CDV, but a conjunctival scrape tested negative with direct IFA. Necropsy also revealed pneumonia with intracytoplasmic and intranuclear viral inclusions. H&E stain. Physical examination findings in dogs with distemper depend on the severity and chronicity of the disease, but include fever (occasionally up to 106°F or 41.1°C), conjunctivitis, serous to mucopurulent ocular and nasal discharges, blepharospasm and photophobia, tonsillar enlargement and hyperemia, tachypnea, and increased lung sounds (see Figure 15-6). Cough may occur during the examination. Dehydration may also be present, especially in dogs with gastrointestinal and severe neurologic signs. Rarely, a measles-like cutaneous rash or pustular dermatitis is evident. Dogs with chronic disease can be thin or emaciated and show hyperkeratosis of the nasal planum and footpads. Puppies with hyperkeratosis, also known as “hardpad,” have thickened, crusty footpads that resemble those of adult dogs (see Figure 15-6). Myoclonus (involuntary twitching of isolated muscle groups) is common and, when mild, is most readily detected when affected puppies are at rest. Other neurologic signs include obtundation, seizures, tremors, opisthotonos, tetraparesis, paraparesis, delayed placing reactions, ataxia, and, less commonly, behavioral abnormalities, compulsive pacing, and vestibular signs such as a head tilt, nystagmus, strabismus, and circling.4 Focal seizures may be localized to the head and jaw, with accompanying foamy hypersalivation (“chewing gum” seizures). Vocalization and apparent blindness can also occur. In addition to conjunctivitis and ocular discharge, an ophthalmologic examination may reveal corneal edema, corneal ulceration, aqueous flare, chorioretinitis, and uveitis, or optic neuritis. Markedly decreased Schirmer tear test results and positive fluorescein tests may be present.18 Optic neuritis appears as a large, pale, fluffy, optic disc with no physiologic depression. Rarely, lameness may be observed. Dogs that have recovered from CDV infection may have evidence of dental abnormalities and enamel hypoplasia (see Figure 15-5); healed chorioretinitis lesions, which appear as hyperreflective circular lesions (“gold medallion lesions”); keratoconjunctivitis sicca; or residual neurologic signs, especially myoclonus. The CBC in dogs with acute distemper most commonly reveals mild anemia and lymphopenia (Table 15-1). However, the lymphocyte count may also be normal, especially with more chronic distemper. Neutropenia, monocytopenia, and thrombocytopenia can occur, and occasionally these are severe. Neutrophilia with a left shift and toxic neutrophils may be present. Rarely, examination of Romanowsky-stained blood smears using light microscopy reveals CDV inclusions in the cytoplasm of circulating erythrocytes and leukocytes (see Figure 15-4, B). Abnormalities on the serum biochemistry panel in dogs with distemper are nonspecific and include electrolyte changes that occur with vomiting and diarrhea such as hyponatremia, hypokalemia, and hypochloridemia (Table 15-2). Hypoalbuminemia and associated hypocalcemia are also common. Mild increases in liver enzyme activities occur in some dogs, which may reflect hypoxia or secondary infections that occur as a result of translocation of intestinal bacteria. CSF analysis may show no clinically significant findings in dogs with distemper, especially when chronic demyelinating encephalitis is present. In some dogs with CNS involvement, a mild to moderate increase in CSF protein concentration is present (usually <100 mg/dL), and there is lymphocytic or monocytic pleocytosis, sometimes with reactivity. Nucleated cell counts are usually less than 50 cells/µL and rarely less than 100 cells/µL.4 Dogs with distemper encephalomyelitis can have normal magnetic resonance imaging findings. Hyperintense lesions and loss of contrast between gray and white matter in the cerebellum and/or the brainstem have been described in T2-weighted images, which correspond to demyelination on histopathology.31 Multifocal T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensities can occur in other locations in the brain, and there may be mild meningeal contrast enhancement.
Canine Distemper Virus Infection
Etiology and Epidemiology
Clinical Features
Physical Examination Findings
Diagnosis
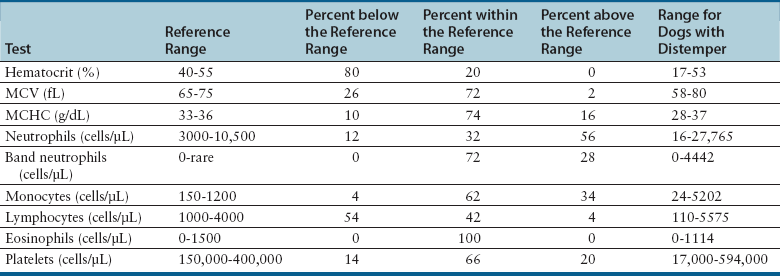
Laboratory Abnormalities
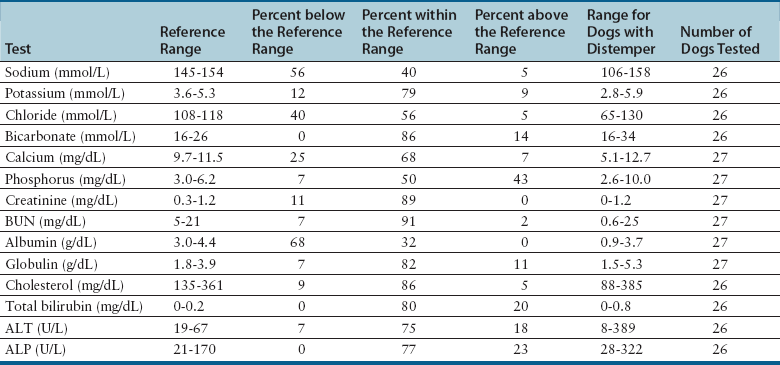
Serum Biochemical Tests
CSF Analysis
Diagnostic Imaging
Magnetic Resonance Imaging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine