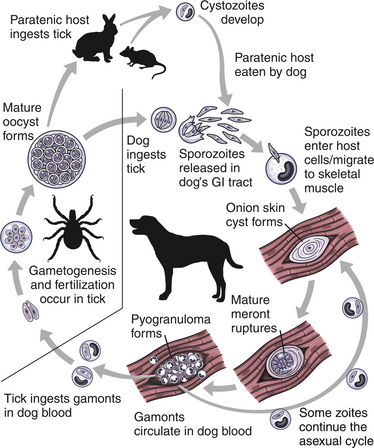
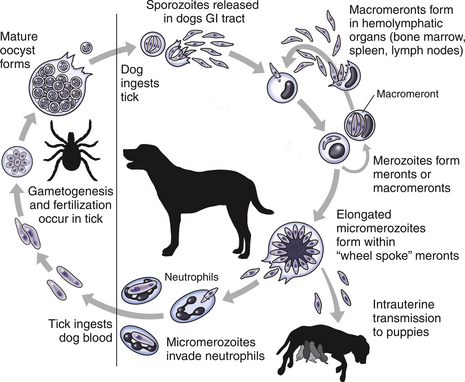
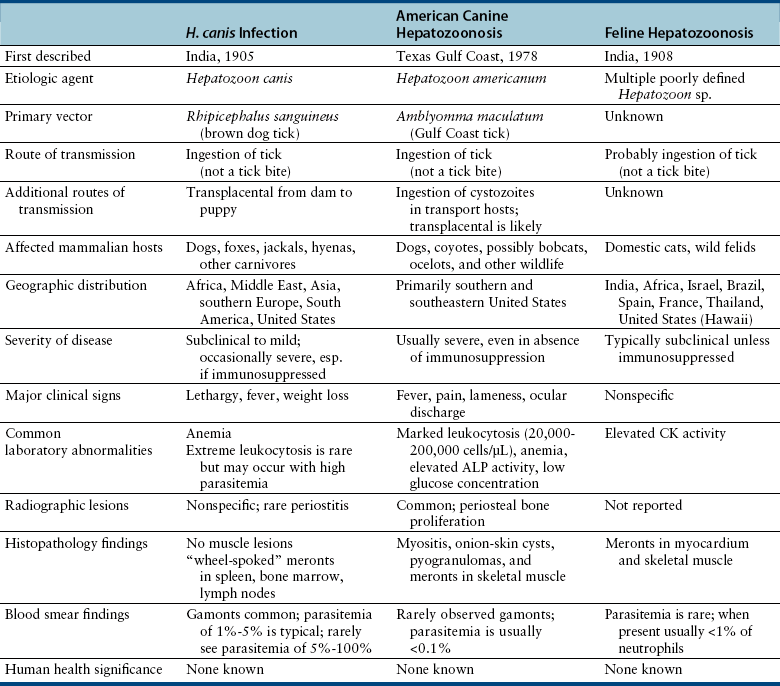
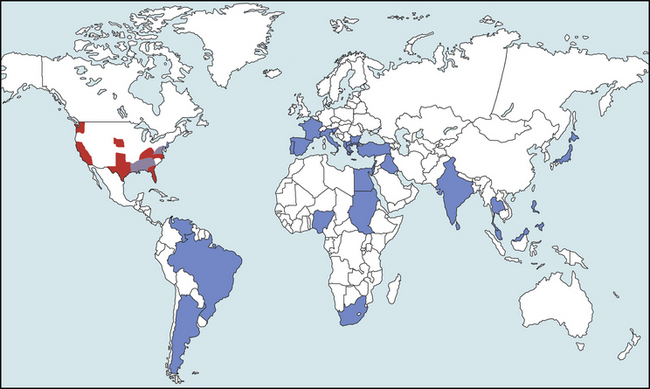
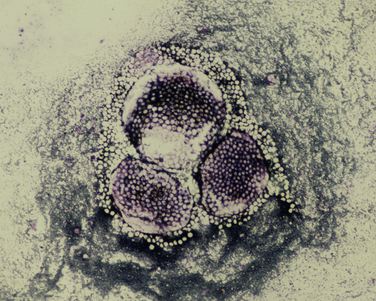
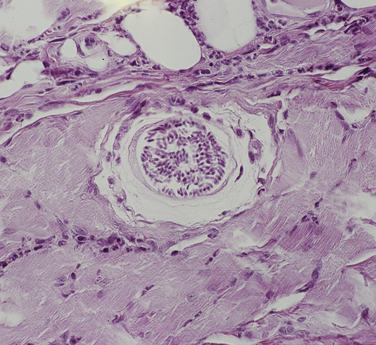
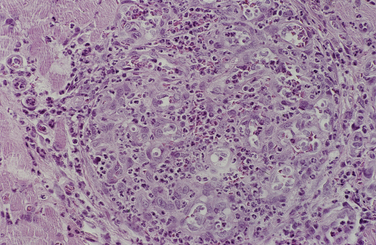
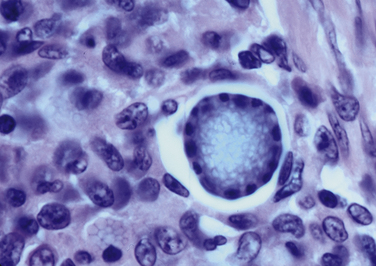
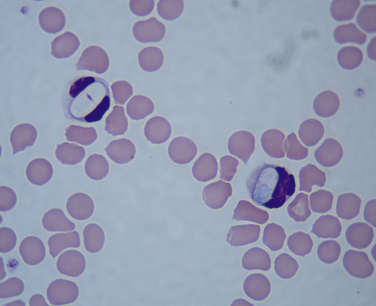
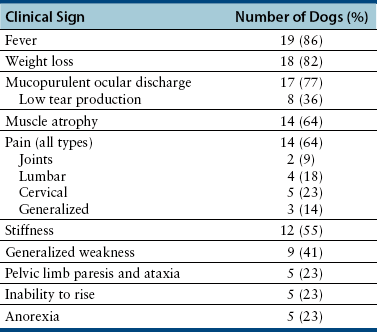
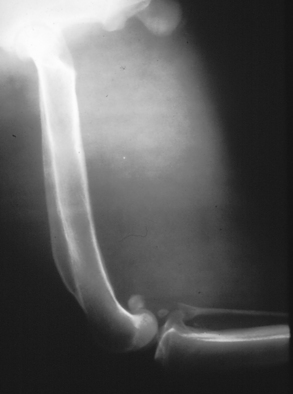
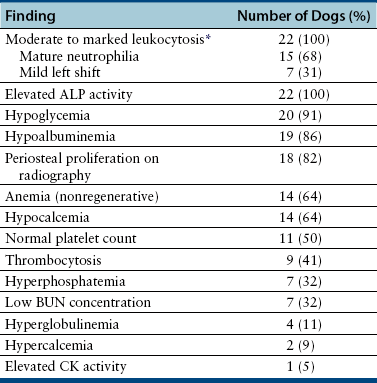
Chapter 77 Apicomplexan protozoal parasites from the genus Hepatozoon include more than 300 species that infect a wide variety of amphibians, reptiles, birds, and mammals.3 Despite its name, canine hepatozoonosis is not a zoonotic disease and rarely affects the liver. Canine hepatozoonosis is caused by one of two species of Hepatozoon currently known to cause disease in dogs, Hepatozoon canis and Hepatozoon americanum (compared in Table 77-1). First described in India in 1905, H. canis has long been known as the agent that infects dogs in many regions of the Old World, including Asia, Africa, southern Europe, and the Middle East, and more recently has been identified in the New World in both North and South America (Figure 77-1).1,3–20 Hepatozoon americanum causes American canine hepatozoonosis, which occurs in dogs primarily in the southeastern and south-central United States. It was first confused with H. canis in 1978 in dogs from the Gulf Coast region of Texas and has subsequently been detected in dogs from Louisiana, Oklahoma, Alabama, Georgia, Florida, and Tennesee.2,21–25 In 1997, it was identified as a distinct species, H. americanum.26,27 Occasional cases have been identified in diverse locations across the United States, including Washington, California, Nebraska, Vermont, and Virginia.17 Because infection is chronic, the infection may be diagnosed in dogs with a history of travel to endemic areas. The DNA of a Hepatozoon felis–like organism was detected in a dog from southern California, but the clinical significance and epidemiology of this organism are unknown.28 TABLE 77-1 Comparison of Hepatozoon canis Infection, American Canine Hepatozoonosis, and Feline Hepatozoonosis FIGURE 77-1 Geographic distribution of reported Hepatozoon canis and Hepatozoon americanum infections. Both species exist in the United States. (Blue, Hepatozoon canis; red, Hepatozoon americanum; purple both species.) Recent reports indicate that H. canis also exists in the United States, although infection with H. americanum is diagnosed much more frequently. H. canis has been identified in dogs from Mississippi, Louisiana, Alabama, Georgia, Oklahoma, and Virginia and in a dog that lived in New Jersey but was born in a Texas shelter.16,17,29 A few dogs have mixed infections with both H. americanum and H. canis. Domestic cats can also be infected by Hepatozoon spp., which include H. felis and possibly H. canis or closely related species (see later section on feline infections and Table 77-1).30,31 Because dogs or other vertebrates serve as intermediate hosts, a vector is needed to complete the sexual phase of the life cycle of Hepatozoon species. In the case of H. americanum, the definitive host is the Gulf Coast tick, Amblyomma maculatum, whereas the definitive host of H. canis is the brown dog tick, Rhipicephalus sanguineus.32,33 In Japan, Haemaphysalis spp. ticks have been implicated as vectors of H. canis, and in Brazil, Amblyomma ovale and Rhipicephalus (Boophilus) microplus are suspected vectors.34–36 As it feeds on an infected dog, the nymphal tick ingests circulating leukocytes that contain gamonts. Within the tick’s gut, the gamonts are freed from the cells and undergo further development before fertilization occurs. The resulting zygote divides through sporogony and develops into an oocyst within the hemocoel of the tick (Figure 77-2). Oocysts mature while the tick molts to the adult stage. In the case of H. americanum, the larval stage of the tick can also become infected, resulting in a nymph that contains viable oocysts capable of transmitting the disease.37 Each mature oocyst contains hundreds of sporocysts, with each sporocyst containing 10 to 26 infective sporozoites. Unlike most tick-borne diseases, hepatozoonosis is not transmitted through the bite of an infected tick but rather by ingestion of an infected tick. This typically occurs when the dog grooms itself but can also occur if the dog feeds on tick-infested prey. Once ingested, exposure to bile in the dog’s intestinal tract results in release of the sporozoites, which penetrate the intestinal epithelial wall and are transported to the target organs and tissues, likely within mononuclear cells. FIGURE 77-2 Hepatozoon americanum oocysts from the hemocoel of an Amblyomma maculatum tick. Hundreds of small, round sporocysts are present within each oocyst. Each sporocyst contains 10 to 26 infective sporozoites. In H. americanum infections, infected cells preferentially travel to skeletal muscle, where each organism develops within its host cell, which becomes lodged between myocytes (Figure 77-3). Concentric layers of mucopolysaccharide are laid down by the host cell to form an “onion-skin cyst,” which may protect the organism from the immune response (Figure 77-4). Merogony occurs within the cyst, and on maturation of the meront, the cyst ruptures, and merozoites are released. This elicits a severe inflammatory response, with recruitment of neutrophils and monocytes to the area. A pyogranuloma develops in the space where the cyst existed (Figure 77-5). Many of these inflammatory cells become infected with a single zoite. Angiogenesis within the pyogranuloma results in a highly vascular structure from which the infected cells can reenter circulation. The intracellular parasites can travel to other target sites, where they continue the asexual reproductive cycle, or they may become gamonts. The gamonts are then ingested by feeding ticks, which completes the life cycle. Some of the onion-skin cysts lie dormant for varying lengths of time; their activation is responsible for the waxing and waning nature of the disease as well as the relapse of clinical signs that can occur months after perceived clinical cure. FIGURE 77-4 Skeletal muscle containing a developing meront of H. americanum. The mucopolysaccharide layers produced by the host cell protect the developing organisms from the host’s immune system. FIGURE 77-5 Pyogranuloma in skeletal muscle of a dog infected with Hepatozoon americanum. Zoites displace the nucleus in several of the inflammatory cells. In H. canis infections, infected cells are carried by lymph or blood to the spleen, bone marrow, lymph nodes, and other organs such as the liver, kidney, and lungs where the organisms divide asexually through merogony (Figure 77-6). Two types of meronts form: the “wheel spoke” meront, which contains 20 to 30 micromerozoites which form around a central round structure (Figure 77-7), and a meront that contains up to four macromerozoites.38 Once released from the mature meront, micromerozoites invade neutrophils and monocytes and become gamonts, which can then be ingested by feeding ticks (Figure 77-8). The larger macromerozoites are believed to be responsible for the production of secondary meronts in the target tissues, which continue the asexual cycle of merogony. Another tissue stage found in dogs with H. canis but not H. americanum infections is a small monozoic cyst that resembles the cystozoite found in transport hosts of other Hepatozoon species.39 FIGURE 77-7 Hepatozoon canis meront in splenic tissue of a dog from Israel demonstrating the typical “wheel spoke” pattern. (Courtesy of Dr. Gad Baneth, Koret School of Veterinary Medicine, Israel.) FIGURE 77-8 Two Hepatozoon canis gamonts in neutrophils on a blood smear. The gamonts of Hepatozoon americanum are nearly identical in appearance to those of H. canis, although they are rarely seen. 1000x magnification. (Courtesy of Dr. Gad Baneth, Koret School of Veterinary Medicine, Israel.) Other modes of transmission also occur in hepatozoonosis. Transplacental transmission from dam to puppies has been documented for H. canis infections and likely occurs in H. americanum infections as well.40 Ingestion of tissue from wild animals or prey may transmit H. americanum.41–43 Laboratory rodents fed H. americanum oocysts developed cystozoites, but they did not develop gamonts or meronts or become ill. Cystozoite-laden rodent tissue fed to naïve dogs resulted in H. americanum infection along with its classical clinical signs. Investigation of a natural outbreak in a pack of hunting beagles revealed that clinical signs of American canine hepatozoonosis arose 4 to 6 weeks after some of the dogs were allowed to consume a wild rabbit carcass, whereas dogs not allowed to consume the rabbit did not develop clinical signs. The predation route of transmission has not yet been proven to occur with H. canis. The geographic distribution of American canine hepatozoonosis aligns closely with the range of the Gulf Coast tick. Although this tick is endemic in the states that surround the Gulf of Mexico, its range is expanding and it has been found as far north as Kansas and Kentucky.44 Larval and nymphal stages of A. maculatum preferentially feed on birds and small rodents. Surveys of wildlife have revealed the presence of Hepatozoon spp. that are related but not identical to H. americanum in small rodents and rabbits.45 Although a reservoir species has not yet been identified, dogs are likely an aberrant, rather than natural, host of H. americanum. H. americanum or closely related species have been identified in coyotes, bobcats, and ocelots.46–49 Because these wild animals were in good physical condition at the time of capture, they may represent a reservoir host in nature. In contrast to adult animals, coyote puppies developed classic signs of H. americanum infection after experimental transmission.50 Dogs are believed to be the natural host reservoir of H. canis. The brown dog tick, which serves as the definitive host of H. canis, is found worldwide, and all three stages preferentially feed on dogs. Life stages of H. canis or morphologically indistinguishable Hepatozoon spp. have been reported in numerous carnivore species around the world, including several species of fox, jackal, African wild dog, hyena, palm civet, cheetah, leopard, lion, and Pallas cat.30 Clinical signs of H. americanum infection are associated with the strong inflammatory response that occurs when meronts rupture, leukocytes are recruited, and pyogranulomas form in skeletal muscle. The earliest lesions occur 3 weeks after infection.51 As the organisms multiply, the infection disseminates, which results in more severe inflammation that waxes and wanes over time. The inflammation causes fever and myositis, which is associated with locomotor abnormalities and hyperesthesia (Table 77-2). The clinical signs can mimic those of meningitis or discospondylitis. Many affected dogs also develop bone lesions that are evident radiographically and resemble lesions seen in hypertrophic osteopathy, except they are proximal rather than distal in distribution (Figure 77-9). It is unknown whether the bone lesions result from nearby localized muscle inflammation or from humoral factors. Dogs usually have a mucopurulent ocular discharge that may be caused by pyogranulomatous inflammation of the extraocular muscles or of the lacrimal gland (Figure 77-10). Return of ocular discharge is frequently the first indication of a relapse following treatment. Dogs with American canine hepatozoonosis frequently maintain a fairly normal appetite; however, weight loss, cachexia, and muscle atrophy occur over time. There may be a history of polyuria and polydipsia that is due to glomerulonephritis or amyloidosis, which can occur secondary to long-standing inflammation. Nephrotic syndrome and thromboembolic complications may ensue. Less common clinical signs include diarrhea, mucosal pallor, cough, abnormal lung sounds, and lymphadenomegaly. TABLE 77-2 Frequency of Clinical Signs in 22 Dogs with American Canine Hepatozoonosis From Vincent-Johnson NA. American canine hepatozoonosis. Vet Clin North Am Small Anim Pract. 2003;33:905-920. Data from Macintire DK, Vincent-Johnson N, Dillon AR, et al. Hepatozoonosis in dogs: 22 cases (1989-1994). J Am Vet Med Assoc. 1997;210:916-922. FIGURE 77-9 Radiograph of the pelvic limb of a dog with Hepatozoon americanum infection. There is smooth periosteal proliferation on the cranial aspect of the femur. (From Vincent-Johnson NA. American canine hepatozoonosis. Vet Clin North Am Small Anim Pract. 2003;33:905-920.) FIGURE 77-10 Rottweiler with Hepatozoon americanum infection. Note the hunched appearance, muscle atrophy, and mucopurulent ocular discharge. Most dogs infected with H. canis have a low level of parasitemia, with less than 5% of circulating leukocytes infected with gamonts.52 These dogs have a limited inflammatory reaction. About 15% of parasitemic dogs have a high level of parasitemia (>800 gamonts/µL). The degree of parasitemia correlates with the severity of clinical signs; the sickest dogs have a level of parasitemia that approaches 100% of infected circulating leukocytes. These dogs may have hepatitis, glomerulonephritis, or pneumonitis in addition to severe anemia, fever, and cachexia. With H. americanum infection, fever (up to 105.6°F or 40.9°C) is common, although because of the waxing and waning nature of the disease, body temperature may be normal at any given time.23 Because of the myositis, dogs are typically evaluated for gait abnormalities, which range from lameness or stiffness to recumbency and an inability to rise. Hyperesthesia is common and may appear as cervical, back, joint, or generalized pain. Lethargy is common, and cachexia and generalized muscle atrophy may be apparent. Mucopurulent ocular discharge is often present. Other ocular lesions seen on occasion include focal retinal scars or hyper-reflectivity, increased retinal pigmentation, papilledema, and uveitis with active inflammatory fundic lesions. Mucosal pallor and lymphadenomegaly may also be present. The most common laboratory abnormality in H. americanum infection is leukocytosis, which is often extreme (Table 77-3). White cell counts are typically 20,000 to 200,000 cells/µL, with reported means of 76,807 and 85,700 cells/µL.23,53 The leukocytosis is due to a mature neutrophilia, although sometimes there is a mild to moderate left shift. A mild normocytic, normochromic nonregenerative anemia is typical. Platelets are usually normal to increased, sometimes with thrombocytosis up to 916,000 platelets/µL. When thrombocytopenia is evident, concurrent tick-borne diseases such as ehrlichiosis should be considered. TABLE 77-3 Frequency of Laboratory and Radiographic Findings in 22 Dogs with American Canine Hepatozoonosis ∗Minimum white cell count was 27,800 cells/µL. From Vincent-Johnson NA. American canine hepatozoonosis. Vet Clin North Am Small Anim Pract. 2003;33:905-920. Data from Macintire DK, Vincent-Johnson N, Dillon AR, et al. Hepatozoonosis in dogs: 22 cases (1989-1994). J Am Vet Med Assoc. 1997;210:916-922. The most common laboratory finding in H. canis infection is anemia, which is usually normocytic, normochromic, and occasionally regenerative.52 Platelets are decreased about one third of the time, but this may be due to concurrent infection with Ehrlichia canis or other disease. The white blood cell count is typically normal in cases with low parasitemia but is elevated in dogs with high parasitemia. Some dogs have neutrophil counts of 50,000 to 150,000/µL with close to 100% of these cells containing gamonts.
Canine and Feline Hepatozoonosis
Etiology and Epidemiology
Clinical Features

Physical Examination Findings
Diagnosis
Complete Blood Count
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine and Feline Hepatozoonosis
Only gold members can continue reading. Log In or Register to continue