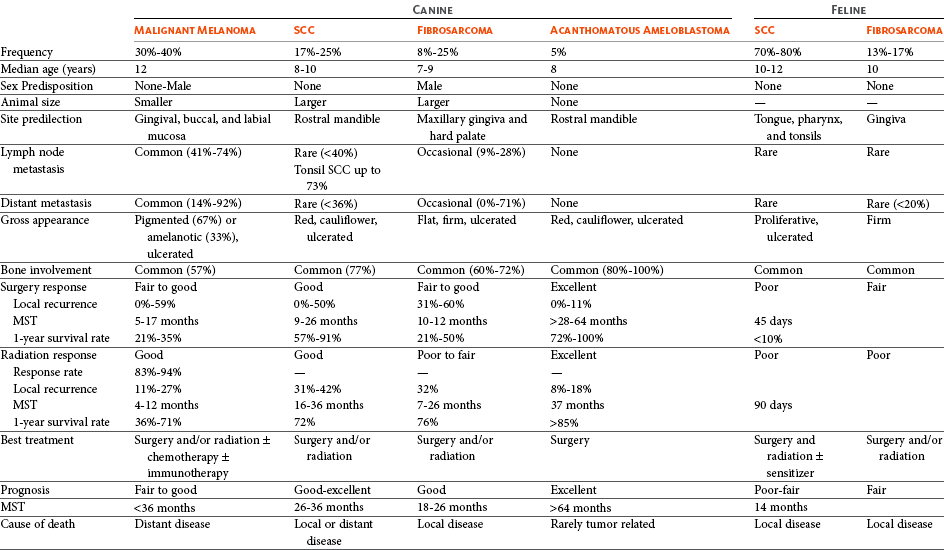
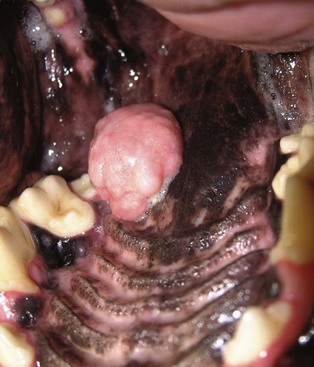
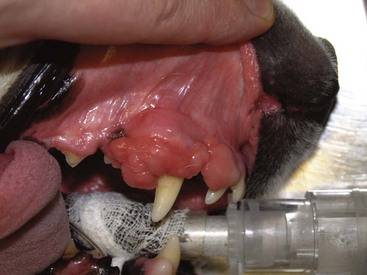
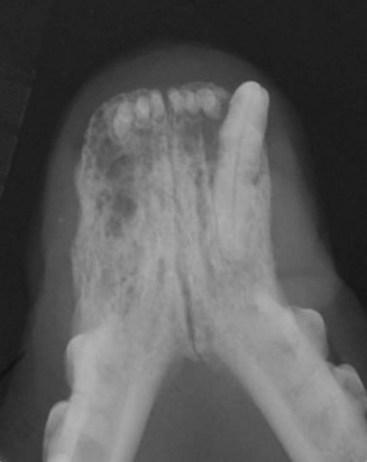
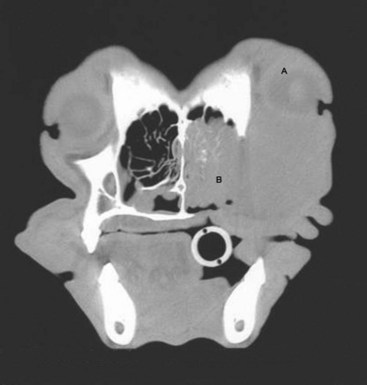
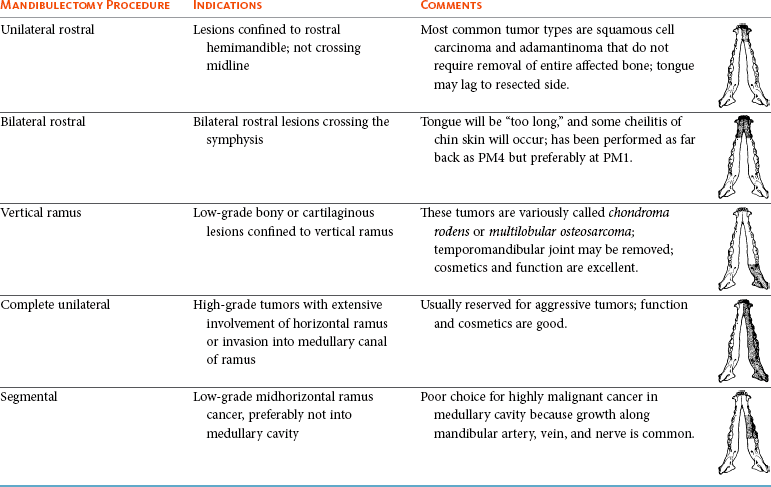
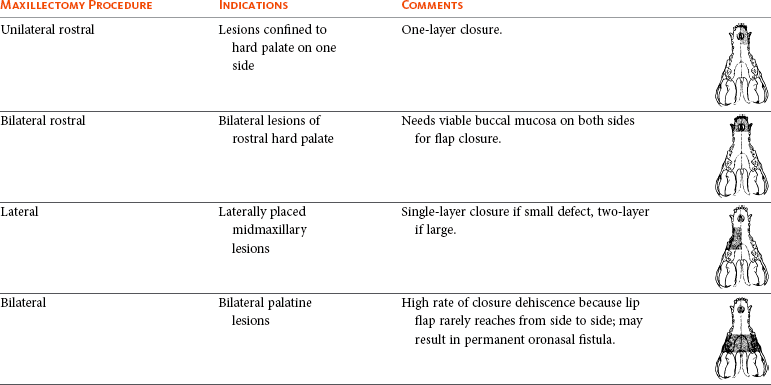
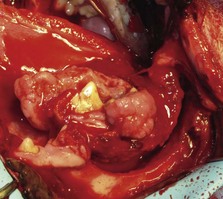
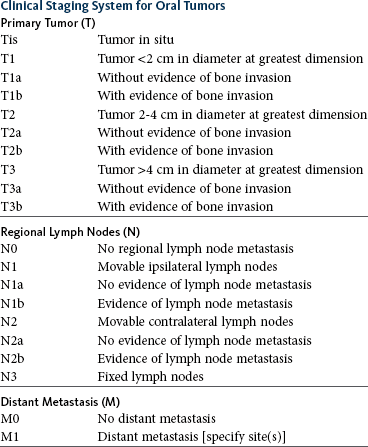
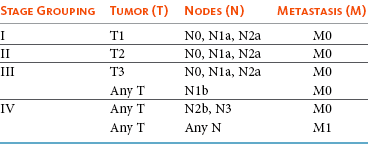
22 Julius M. Liptak and Stephen J. Withrow Collectively, oral cancer accounts for 6% to 7% of canine cancer and is the fourth most common cancer overall.1,2 In the cat, it accounts for 3% of all cancers.3 Oropharyngeal cancer is 2.6 times more common in dogs than cats, and male dogs have a 2.4 times greater risk of developing oropharyngeal malignancy compared to female dogs.4,5 A male sex predisposition has also been reported for dogs with malignant melanoma and tonsillar squamous cell carcinoma (SCC).6,7 Dog breeds with the highest risk of developing oropharyngeal cancer include the cocker spaniel, German shepherd dog, German shorthaired pointer, Weimaraner, golden retriever, Gordon setter, miniature poodle, Chow Chow, and Boxer.6,8,9 In one study, German shepherd dogs and Boxers had a decreased risk of developing oral melanoma.9 In dogs, the most common malignant oral tumors are, in descending order, malignant melanoma, SCC, and fibrosarcoma,10–21 although in other studies SCC is more common than malignant melanoma.2 SCC is the most common oropharyngeal cancer in cats, followed by fibrosarcoma, which accounts for 13% of feline oral tumors.3 Other malignant oral tumors in dogs include osteosarcoma, chondrosarcoma, anaplastic sarcoma, multilobular osteochondrosarcoma, intraosseous carcinoma, myxosarcoma, hemangiosarcoma, lymphoma, mast cell tumor, and transmissible venereal tumor.10–24 Tumors or tumorlike lesions of unusual sites, types, and biologic behavior (e.g., tonsillar SCC, tongue, malignancy of young dogs, viral papillomatosis, canine and feline eosinophilic granuloma complex, epulis, inductive fibroameloblastoma, and nasopharyngeal polyps) will be covered at the end of this chapter. A general summary of the common oral tumors is found in Table 22-1. In comparison to other malignant oral tumors, malignant melanoma tends to occur in smaller body weight dogs. Cocker spaniel, miniature poodle, Anatolian sheepdog, Gordon setter, Chow Chow, and golden retriever are overrepresented breeds.9 A male predisposition has been reported,7 but this is not consistent.9 The mean age at presentation is 11.4 years.9 Malignant melanoma occurs in cats but is uncommon.25 Malignant melanoma can present a confusing histopathologic picture if the tumor or the biopsy section does not contain melanin, and amelanotic melanomas represent one-third of all cases. A histopathologic diagnosis of undifferentiated or anaplastic sarcoma or even epithelial cancer should be viewed with suspicion for possible reclassification as melanoma. Melan A is an immunohistochemical stain with a moderate sensitivity and specificity for the diagnosis of melanoma in dogs and can be used to differentiate melanoma from other poorly differentiated oral tumors and may be helpful in differentiation.9 Melanoma of the oral cavity is a highly malignant tumor with frequent metastasis to the regional lymph nodes and then the lungs.7,26,27 The metastatic rate is site, size, and stage dependent and reported in up to 80% of dogs.* The World Health Organization (WHO) clinical staging system for oral tumors in dogs may have prognostic significance in dogs with oral melanoma (Table 22-2).7,26,31,36–38 Malignant melanoma is a highly immunogenic tumor, and molecular and immunomodulatory approaches to treatment are active areas of research.33,34,39–47 A review of the biology and molecular mechanisms of canine melanoma development and progression is provided in Chapter 19.48,49 Table 22-2 Clinical Staging (TNM) of Oral Tumors in Dogs and Cats Data from Owen LN: TNM classification of tumors in domestic animals, Geneva, 1980, WHO. SCC is the most common oral tumor in cats and the second most common in dogs.1,3,18–21 In cats, the risk of developing oral SCC is significantly increased by over 3.5-fold with the use of flea collars and high intake of either canned food in general or canned tuna fish specifically.50 Exposure to household smoke increases the risk of oral SCC by twofold in cats,50 and although this was not statistically significant, smoke exposure is associated with a significant increase in expression of p53 in SCC lesions compared to cats with oral SCC not exposed to environmental smoke.51 For this reason, mutations of p53 may be involved in the development and progression of smoke-related oral SCC in cats. SCC frequently invades bone in both cats and dogs, and bone invasion is usually severe and extensive in the cat. Increased tumor expression of parathyroid hormone–related protein in cats with oral SCC may play a role in bone resorption and tumor invasion.52 Paraneoplastic hypercalcemia has also been reported in two cats with oral SCC.53 Metastasis in the cat is rare and the true incidence is unknown because so few cats have had their local disease controlled; thus an accurate estimate of the metastatic potential has not been confirmed. The metastatic rate for non–tonsillar SCC in dogs is approximately 20%,32 but the metastatic risk is site dependent—the rostral oral cavity has a low metastatic rate and the caudal tongue and tonsil have a high metastatic potential. Oral fibrosarcoma is the second most common oral tumor in cats and the third most common in dogs.1,3,18–21,54 In dogs, oral fibrosarcoma tends to occur in large breed dogs, particularly golden and Labrador retrievers; at a younger age, with a median of 7.3 to 8.6 years; and with a possible male predisposition. Oral fibrosarcoma will often look surprisingly benign histologically and, even with large biopsy samples, the pathologist can find it difficult to differentiate fibroma from low-grade fibrosarcoma. This syndrome, which is common on the hard palate (Figure 22-1) and maxillary arcade between the canine and carnassial teeth of large-breed dogs, has been termed histologically low-grade but biologically high-grade fibrosarcoma.54 Even with a biopsy result suggesting fibroma or low-grade fibrosarcoma, the treatment should be aggressive, especially if the cancer is rapidly growing, recurrent, or invading bone. Fibrosarcoma is locally invasive but metastasizes to the lungs and occasionally regional lymph nodes in less than 30% of dogs.10,18–21,32 Epulides are benign gingival proliferations arising from the periodontal ligament and appear similar to gingival hyperplasia (Figure 22-2). Three types of epulides have previously been described in the dog: acanthomatous, fibromatous, and ossifying.56–58 However, the terminology for these tumors has changed; acanthomatous epulis is now termed acanthomatous ameloblastoma and peripheral odontogenic fibroma is the preferred nomenclature for fibrous and ossifying epulides.59 Epulides are relatively common in dogs but rare in cats. Multiple epulides have been described in cats with 50% of cases occurring in cats younger than 3 years.55 The mean age at presentation for dogs with peripheral odontogenic fibromas is 8 to 9 years, and a male predisposition has been reported in one study.57,58 Peripheral odontogenic fibromas are slow-growing, firm masses that are usually covered by intact epithelium. They have a predilection for the maxilla rostral to the third premolar teeth.58,59 Acanthomatous ameloblastoma has an aggressive local behavior and frequently invades bone of the underlying mandible or maxilla. Shetland and Old English sheepdogs are predisposed.57,58 The mean age at presentation is 7 to 10 years, and a sex predisposition is unlikely with three studies reporting conflicting results.57,60,61 The rostral mandible is the most common site.60 They do not metastasize. Acanthomatous ameloblastoma is the preferred term, but some pathologists will refer to these tumors by their previous terminology of acanthomatous epulis or adamantinoma.56 Most cats and dogs with oral cancer present with a mass in the mouth noticed by the owner. Cancer in the caudal pharynx, however, is rarely seen by the owner, and the animal will present for signs of increased salivation, exophthalmos or facial swelling, epistaxis, weight loss, halitosis, bloody oral discharge, dysphagia or pain on opening the mouth, or occasionally cervical lymphadenopathy (especially SCC of the tonsil).18–21,62 Loose teeth, especially in an animal with generally good dentition, should alert the clinician to possible underlying neoplastic bone lysis (Figure 22-3), especially in the cat.63 Although paraneoplastic syndromes associated with oral tumors are rare, hypercalcemia has been reported in two cats with oral SCC51 and hyperglycemia in a cat with a gingival vascular hamartoma.64 Cancers that are adherent to bone, other than peripheral odontogenic fibromas, should have regional radiographs taken under general anesthesia. Regional radiographs include open mouth, intraoral, oblique lateral, and ventrodorsal or dorsoventral projections.65 Bone lysis is not radiographically evident until 40% or more of the cortex is destroyed (Figure 22-4). However, apparently normal radiographs do not exclude bone invasion. This evaluation will assist in determining clinical staging information and the extent of resection when surgery is indicated. Computed tomography (CT) or magnetic resonance imaging (MRI) can be a very valuable staging tool, especially for evaluation of bone invasion and possible tumor extension into the nasal cavity or in the caudal pharynx and orbit, and is preferred to regional radiographs when available (Figure 22-5).66 Regional lymph nodes should be carefully palpated for enlargement or asymmetry. However, caution should be exercised when making clinical judgments of whether neoplastic involvement of regional lymph nodes is present. Lymph node size is not an accurate predictor of metastasis. In one study of 100 dogs with oral melanoma, 40% of dogs with normal-sized lymph nodes had metastasis and 49% of dogs with enlarged lymph nodes did not have metastasis.27 The regional lymph nodes include the mandibular, parotid, and medial retropharyngeal lymph nodes; however, the parotid and medial retropharyngeal lymph nodes are normally not palpable.67 Furthermore, only 55% of 31 cats and dogs with metastasis to the regional lymph nodes had metastasis to the mandibular lymph node.68 Lymphoscintigraphy or contrast-enhanced ultrasonography can be used to detect the sentinel lymph nodes and guide lymph node aspirates.69 Lymph node aspiration should be performed in all animals with oral tumors, regardless of the size or degree of fixation of the lymph nodes.27,68 En bloc resection of the regional lymph nodes has been described and, although the therapeutic benefit of this approach is unknown, it may provide valuable staging information.67,68 Based on these diagnostic steps, oral tumors are then clinically staged according to the WHO staging scheme (see Table 22-2).36 Surgery and RT are the most common treatments used for the local control of oral tumors. Surgical resection is the most economic, expeditious, and curative treatment. The type of oral surgery depends on tumor histology and location. Except for peripheral odontogenic fibromas, most oral tumors have some underlying bone involvement and surgical resection should include bony margins to increase the likelihood of local tumor control. More aggressive surgeries such as mandibulectomy, maxillectomy, and orbitectomy are generally well tolerated by cats and dogs. These procedures are indicated for all aggressive and/or invasive oral tumors, particularly lesions with extensive bone invasion, with poor sensitivity to RT, or that are too large for cryosurgery (Tables 22-3 and 22-4).11–21,70–73 Margins of at least 2 cm are necessary for malignant cancers such as SCC, malignant melanoma, and fibrosarcoma in the dog. If possible, SCC in the cat should be treated with surgical margins greater than 2 cm because of high local recurrence rates. Bone reconstruction following bony resection has been described but is rarely necessary.13,74–76 Rostral and segmental resections (e.g., mandibulectomy and maxillectomy) may be sufficient for benign lesions and rostral SCC in dogs. Rim resections with a biradial oscillating saw, in which the ventral cortex of the mandible is preserved, may be possible for small benign tumors localized to the alveolar margin of the mandible (Figure 22-6).77 Larger resections, including hemimandibulectomy, hemimaxillectomy, orbitectomy, and radical maxillectomy, are necessary for more aggressive tumors, especially fibrosarcoma, and malignant tumors with a more caudal location.11–21,70–72 Although these large resections carry some morbidity, owner satisfaction with the cosmetic and functional outcomes is in excess of 85%.11–21,71,73,78 Cosmesis is usually very good following most mandibulectomy and maxillectomy procedures (Figure 22-7) but can be challenging with aggressive bilateral rostral mandibulectomies and radical maxillectomies.11–21,70–72 Blood loss and hypotension are the most common intraoperative complications, particularly during caudal or aggressive maxillectomy procedures.19,71 Postoperative complications include incisional dehiscence, epistaxis, increased salivation, mandibular drift and malocclusion, and difficulty prehending food, particularly after bilateral rostral mandibulectomy caudal to the second premolar teeth.13–21,53,71 Elastic training, consisting of an orthodontic elastic rubber chain between an orthodontic button on the lingual aspect of the intact mandible tooth and buccal aspect of the maxillary fourth premolar tooth, has been described to maintain occlusion and prevent mandibular drift following mandibulectomy in dogs.79 Enteral feeding tubes are not usually required following oral surgery in dogs; however, they are recommended for cats treated with any type of mandibulectomy because eating can be difficult for 2 to 4 months following surgery.53,78 Local disease control is the goal of treatment for most animals with oral tumors. Regional lymph node resection has been described in cats and dogs; although it adds to clinical staging information, its effectiveness in controlling local and metastatic disease is unknown.67,68 RT can be effective for locoregional control of oral tumors. RT can be used as a primary treatment, with either palliative or curative intent, or as an adjunct for incompletely resected tumors or tumors with an aggressive local behavior, such as oral fibrosarcoma. Malignant melanoma, canine oral SCC, and some benign tumors, such as the acanthomatous ameloblastoma, are known to be radiation responsive, and RT should be considered in the primary treatment of these tumors.31–33,80,81 For canine oral SCC, dental tumors, and fibrosarcoma, daily and alternate day protocols have been described consisting of 2.7 Gy to 4.2 Gy per fraction with a total dose ranging from 48 Gy to 57 Gy.32,82 Tumor control is better for smaller benign and malignant lesions (T1 and T2 tumors) treated with radiation alone.31,32,80 Local tumor control and survival time may be improved by combining RT with surgery and/or chemotherapy, especially for tumors considered radiation resistant, such as canine oral fibrosarcoma and feline oral SCC.53,83–88 Radiation sensitizers, such as etanidazole and gemcitabine, may improve response rates in cats with oral SCC, and platinum drugs have been used as radiation sensitizers in dogs with oral melanoma.28,37,84,87,88 However, gemcitabine is not recommended as a radiosensitizer in cats because of significant hematologic and local tissue toxicities.89 Oral melanoma is moderately responsive to coarse fractionation protocols. Four different hypofractionated radiation protocols have been described: (1) three weekly 8- to 10-Gy fractions for a total dose of 24 to 30 Gy,30,37 (2) four weekly fractions of 9 Gy for a total dose of 36 Gy,31,37 (3) six weekly 6-Gy fractions for a total dose of 36 Gy,28 and (4) eight weekly 6-Gy fractions for a total dose of 48 Gy.90 In humans, the effect of total radiation dose is controversial, but fraction size does have an impact on response rates. Doses greater than 4 Gy per fraction are recommended as response rates are significantly better with fractions greater than 8 Gy compared to less than 4 Gy.91 However, in one study of dogs with oral melanoma comparing two hypofractionated protocols of 9 to 10 Gy per fraction to a fully fractionated protocol of 2 to 4 Gy per fraction, there were no significant differences in either local recurrence rates or survival time.37 Coarse fractionation of oral melanoma has also been described in five cats with limited success, including one complete response and two partial responses.25 Acute effects are common but self-limiting. These include alopecia and moist desquamation, oral mucositis, dysphagia, and ocular changes, such as blepharitis, conjunctivitis, keratitis, and uveitis.32,80,92–94 The acute effects of coarse fractionation are less than experienced with the full-course protocols used for oral SCC and dental tumors and usually resolve rapidly.31 Late complications are rare, occurring in less than 5% of cases, but can include permanent alopecia, skin fibrosis, bone necrosis and oronasal fistula formation, development of a second malignancy within the radiation field, keratoconjunctivitis sicca, cataract formation, xerostomia, and retinal atrophy.32,61,80,95 Orthovoltage radiation may be associated with a higher incidence of second malignancies and bone necrosis than megavoltage irradiation.32,61,80 Hyperthermia offers no advantage over cryosurgery or surgery if it is used alone. In fact, bone penetration is less reproducible with heat versus cold treatment. Hyperthermia at moderate temperatures (42 to 43°C) has been used as an adjunct to irradiation.29,96,97 The major problem with most oral tumors is control of local disease. However, chemotherapy is indicated for some tumors with higher metastatic potential, especially oral melanoma in dogs and tonsillar SCC in cats and dogs. Expression of cyclooxygenase 2 (COX-2) has been noted in feline oral SCC98; however, nonsteroidal antiinflammatory drugs (NSAIDs) such as piroxicam have not been effective in the management of this disease in cats in preliminary studies.99 Piroxicam does appear to have some effect against oral SCC in dogs,100 and response rate is improved when piroxicam is combined with either cisplatin or carboplatin.101,102 Liposome-encapsulated cisplatin is not effective in cats with oral SCC,103 but mitoxantrone, in combination with RT, has shown some potential in a limited number of cats with good local responses and durable remission.85,86 The platinum drugs show the most promise, albeit modest, in treating dogs with oral melanoma, including intralesional cisplatin104 and systemic carboplatin.105 Measurable responses to melphalan have also been reported.106 Malignant melanoma is a highly immunogenic tumor. The use of immunomodulatory agents is an emerging and exciting approach for the adjunctive management of dogs with oral melanoma. A thorough discussion of the immunotherapeutic approach to management of canine melanoma is provided in Chapter 19. Clinical series of over 500 dogs with various oral malignancies treated with either mandibulectomy or maxillectomy have been described.11–21,70–72 The majority of cases were treated with surgery alone. Unfortunately, the methods of reporting and outcome results vary with each paper, but an attempt to combine cases by tumor type and outcome is shown in Tables 22-5 and 22-6. Overall, the lowest rates of local tumor recurrence and best survival times are reported in dogs with acanthomatous ameloblastoma and SCC, whereas fibrosarcoma and malignant melanoma are associated with the poorest results.11–21 Most of these reports suggest that histologically complete resection, smaller diameter, and a rostral location are favorable prognostic factors. In two studies of 142 dogs treated with either mandibulectomy or maxillectomy, tumor-related deaths were 10 to 21 times more likely with malignant tumors, up to 5 times more likely with tumors located caudal to the canine teeth, and 2 to 4 times more likely following incomplete resection.20,21 Rostral locations are usually detected at an earlier stage and are more likely to be resectable with complete surgical margins. Local tumor recurrence is more frequent following incomplete resection, with 15% to 22% and 62% to 65% of tumors recurring following complete and incomplete excision, respectively.20,21 Recurrent disease negatively impacts survival time because further treatment is more difficult and the response to treatment is poorer.35 Fibrosarcoma continues to have an unacceptable local recurrence rate and needs to be addressed with wider resections or other adjuvant therapies, such as postoperative radiation.82 On the other hand, melanoma is controlled locally in 75% of cases, but metastatic disease requires more effective adjuvant therapy, such as RT, chemotherapy, or immunotherapy. For dogs treated with megavoltage radiation, tumor type and tumor size are important factors in local tumor control for both benign and malignant oral tumors. As noted previously, acanthomatous ameloblastoma and SCC in dogs are radiation sensitive. Local recurrence is reported in 30% of oral tumors regardless of treatment, but recurrence is a function of tumor size. Compared to small tumors (T1: <2-cm diameter), recurrence is 3 times more likely in T2 tumors (2- to 4-cm diameter) and up to 8 times more likely in T3 tumors (>4-cm diameter).32,80 Tumor size is also associated with survival in dogs with malignant oral tumors, with 3-year progression-free survival (PFS) rates of 55%, 32%, and 20% for T1, T2, and T3 tumors, respectively.32 The prognosis for dogs with oral melanoma is guarded. Metastatic disease is the most common cause of death with metastasis to the lungs reported in 14% to 67% of dogs.* Surgery or RT can provide good local control, but strategies to manage the high metastatic potential such as chemotherapy and immunotherapy require further investigation. Surgery is the most common treatment for management of the local tumor. The local tumor recurrence rate varies from 22% following mandibulectomy to 48% after maxillectomy.7,18,19 The median survival time (MST) for dogs with malignant melanoma treated with surgery alone varies from 150 to 318 days with 1-year survival rates less than 35%.† Regardless, tumor control and survival time are significantly better when surgery is included in the treatment plan.38 In comparison, the MST for untreated dogs with oral melanoma is 65 days.35 Variables known to have prognostic significance in dogs treated with surgery alone or in combination with other modalities include tumor size, clinical stage, and ability of the first treatment to achieve local control.‡ Dogs with tumors less than 2-cm in diameter have a MST of 511 days compared to 164 days for dogs with tumors greater than 2-cm diameter or lymph node metastasis.33 MSTs are significantly shorter for dogs with recurrent oral malignant melanomas compared to dogs with previously untreated oral melanomas.26,35 Oral melanoma is responsive to hypofractionated RT protocols. Response rates are excellent with 83% to 100% of tumors responding and a complete response observed in up to 70% of melanomas.28,30–32,90 Local recurrence is reported in 15% to 26% of dogs experiencing a complete response with a median time to local recurrence of 139 days.28,30–32 Progressive local disease was observed in all dogs that did not achieve a complete response in one study.30 The most common cause of death is metastasis and this is reported in 58% of dogs with a median time of metastasis of 311 days.28 The MST for dogs treated with RT is 211 to 363 days, with a 1-year survival rate of 36% to 48% and a 2-year survival rate of 21%.28,30–32,37 Local tumor control and survival time are significantly improved with rostral tumor location, smaller tumor volume, no radiographic evidence of bone lysis, and postoperative irradiation of microscopic disease.31,32,37 The risk factors associated with poor outcomes in dogs with melanoma include nonrostral location, bone lysis, and macroscopic disease, and in one series of 140 dogs with oral melanoma, the MST was 21 months if none of these risk factors were present compared to a MST of 11 months with one risk factor, 5 months with two risk factors, and 3 months with all three risk factors.37 Tumor size is important with median PFS for dogs with T1 oral melanomas of 19 months compared to less than 7 months for T2 and T3 tumors.32 Hypofractionated RT has also been described in five cats with oral melanoma, resulting in a 60% response rate and MST of 146 days (range: 66 to 224 days).25 Chemotherapy or immunotherapy is indicated in the adjunctive management of dogs with oral melanoma because of the high metastatic risk. A thorough discussion of malignant melanoma and its prognosis following definitive treatment with surgery, RT, chemotherapy, and/or immunomodulatory agents is provided in Chapter 19. The location of malignant melanoma may also have some prognostic significance. Melanomas of the lip and tongue have a lower metastatic rate and survival is more dependent on local control of the tumor. In one series of 60 dogs with oral melanomas at various sites treated with combinations of surgery, RT, chemotherapy, and immunotherapy, the MST for dogs with lip melanomas was 580 days and was not reached and greater than 551 days for dogs with tongue melanomas.7 In comparison, the MST was 319 days for maxillary melanomas and 330 days for melanomas of the hard palate.7 In another study, only 5% of 64 dogs with well-differentiated melanomas of the mucous membranes of the lips and oral cavities treated with surgery alone had died from tumor-related reasons with an overall MST of 34 months.109 This improved prognosis may reflect the location of these lesions (lip compared to oral cavity) or the degree of differentiation. Nuclear atypia and mitotic index has also been shown to be prognostic in dogs with oral malignant melanomas.110 The prognosis for dogs with oral SCC is good, particularly for rostral tumor locations. Local tumor control is usually the most important challenge, although metastasis to the regional lymph nodes is reported in up to 10% of dogs and to the lungs in 3% to 36% of dogs.32 In contrast, SCC of the tonsils and base of the tongue are highly metastatic, with metastasis reported in up to 73% of dogs, and locoregional recurrence is common.111–113 Surgery and RT can both be used for locoregional control of oral SCC in dogs. Photodynamic therapy has also been reported with fair-to-good results in 11 dogs with smaller oral SCC.114 Surgery is the most common treatment for non–tonsillar SCC.11 Following mandibulectomy, the local recurrence rate is 10% and the MST varies from 19 to 26 months with a 91% 1-year survival time.18 In comparison, the local recurrence rate is 29% after maxillectomy with a MST of 10 to 19 months and a 1-year survival rate of 57%.19 The higher local control and survival rates with mandibular resections probably result because the rostral mandible is the most common location for oral SCC in dogs, and complete surgical resection is more likely for rostral locations. Full-course RT, either alone or as an adjunct following incomplete surgical resection, is also a successful treatment modality for the management of oral SCC in dogs.32,115,116 The local tumor recurrence rate is 31%. The MST for RT alone is 15 to 16 months and increases to 34 months when combined with surgery.115,116 In one series of 39 dogs with oral SCC, the overall median PFS time was 36 months with 1- and 3-year PFS rates of 72% and 55%, respectively.32 Local tumor control was more successful with smaller lesions; the median PFS time for T1 tumors (<2-cm diameter) was not reached and greater than 68 months compared to 28 months for T2 tumors (2- to 4-cm diameter) and 8 months for dogs with T3 tumors (>4-cm diameter).32 Additional favorable prognostic factors for dogs receiving orthovoltage irradiation include rostral tumor location, maxillary SCC, and young age.115 Younger age is also favorable for dogs treated with megavoltage radiation (< 9 years of age—1080 days; > 9 years of age—315 days).116 Chemotherapy is indicated for dogs with metastatic disease, dogs with bulky disease, and when owners decline surgery and RT. However, as the metastatic potential of oral SCC in dogs is relatively low, the role of chemotherapy in minimizing the risk of metastatic disease is unknown. In a series of 17 dogs treated with piroxicam alone, the response rate was 17%, with one complete response and two partial responses.100 The median progression-free interval for dogs responding to piroxicam was 180 days and significantly longer than the 102 days for dogs with stable disease.100 The outcome is better when piroxicam is combined with either cisplatin or carboplatin. In a series of nine dogs treated with piroxicam and cisplatin, the overall MST was 237 days, with the 56% of dogs responding to this chemotherapy protocol having a significantly better outcome than nonresponders with a MST of 272 days compared to 116 days.101 However, renal toxicity was reported in 41% of dogs in this study and such toxicities limit the clinical usefulness of this protocol. In another small series of seven dogs with T3 oral SCC treated with piroxicam and carboplatin, a complete response was observed in 57% of dogs and this response was sustained in all dogs at the median follow-up time of 534 days.102 Novel therapies under investigation include the combination of intralesional bleomycin and feline interleukin-12 (IL-12) DNA with translesional electroporation.107 The prognosis for cats with oral SCC is poor.14,62,117,118 There is no known effective treatment that consistently results in durable control or survival. Local control is the most challenging problem. In one series of 52 cats, the 1-year survival rate was less than 10%, with MSTs of 3 months or less for surgery alone, surgery and RT, RT and low-dose chemotherapy, or RT and hyperthermia.62 However, 42% of these cats had SCC involving the tongue, pharynx, or tonsils. In another series of 54 cats treated in general practice, the MST was 44 days with a 9.5% 1-year survival rate.119 The oncologic outcome may be better for cats with mandibular SCC. The MST for seven cats treated with a combination of mandibulectomy and RT was 14 months with a 1-year survival rate of 57%.53 Local recurrence was the cause of failure in 86% of these cats between 3 to 36 months after therapy. In another series of 22 cats treated with mandibulectomy alone, the median disease-free interval (DFI) was 340 days.78 Tumor location and extent of resection had prognostic importance with a MST of 911 days for rostral tumors, 217 days following hemimandibulectomy, and 192 days when more than 50% of the mandible was resected.78 Expansile, blastic, and discrete lesions are often more resectable than invasive, lytic, and ill-defined lesions in the experience of the authors. The use of esophagostomy or gastrostomy tubes may be necessary to provide supplemental nutrition in these cats for up to 4 months postoperatively.78 RT alone is generally considered ineffective in the management of cats with oral SCC. In nine cats treated with an accelerated radiation protocol (14 fractions of 3.5 Gy delivered twice daily for 9 days), the overall MST was 86 days and, although not significant, the MST for cats with a complete response was 298 days.81 The combination of RT with radiation sensitizers or chemotherapy improves response rates and survival times. Using the same accelerated radiation protocol with carboplatin resulted in a MST of 163 days in 14 cats.88 Intratumoral etanidazole, a hypoxic cell sensitizer, resulted in a 100% partial response rate in nine cats completing the RT course with a median decrease in tumor size of 70% and a MST of 116 days.84 Gemcitabine was used at low doses as a radiation sensitizer in eight cats with oral SCC with an overall response rate of 75%, including two cats with complete responses, for a median duration of 43 days and a MST of 112 days.87 However, gemcitabine is not recommended as a radiosensitizer in cats because of significant hematologic and local tissue toxicities.89 The combination of RT with mitoxantrone holds some promise because, in two series of 18 cats, a complete response was observed in 73% with a median duration of response of 138 to 170 days and an MST of 184 days.85,86 Palliative radiation protocols, consisting of 8 Gy fractions on days 0, 7, and 21, are not recommended because of poor disease control and radiation-induced adverse effects.120 Localized irradiation with strontium-90 may be effective for selected cats with very superficial disease.121 Chemotherapy appears ineffective in the management of cats with oral SCC. No responses were observed in 18 cats treated with liposome-encapsulated cisplatin or 13 cats treated with piroxicam.99,103 In one study, the administration of a NSAID improved survival times in cats with oral SCC.119 The prognosis for dogs with oral fibrosarcoma is guarded. These are locally aggressive tumors and local control is more problematic than metastasis. Metastasis is reported to the regional lymph nodes in 19% to 22% of dogs and to the lungs in up to 27% of dogs.18,19,32 Multimodality treatment of local disease appears to afford the best survival rates, with combinations of surgery and RT or RT and hyperthermia.29,78 Surgery is the most common treatment for oral fibrosarcoma. The median DFI for five cats treated with mandibulectomy was 859 days.78 Following mandibulectomy, local recurrence is reported in 59% of dogs with a MST of 11 months and a 1-year survival rate of 50%.18 The outcome is similar following maxillectomy, with local recurrence in 40% of dogs and a MST of 12 months.19 One-year survival rates rarely exceed 50% with surgery alone.11–21 The combination of surgery and RT provides the best opportunity to control local disease. Oral fibrosarcomas are considered radiation resistant.83,122,123 The mean survival time of 17 dogs treated with RT alone was only 7 months.83 RT combined with regional hyperthermia improved local control rates to 50% at 1 year in a series of 10 cases.29 When RT is used as an adjunct to surgical resection, local tumor recurrence was reported in 32% of dogs overall and the MST increased to 18 to 26 months with a 1-year PFS rate of 76%.32,82 A smaller tumor size improves the outcome following RT, with a median PFS time of 45 months for dogs with T1 tumors compared to 31 months and 7 months for T2 and T3 tumors, respectively.32 Osteosarcoma of axial sites is less common than appendicular osteosarcoma and represents approximately 25% of all cases.124 Of the axial osteosarcomas, the mandible and maxilla are involved in 27% and 16% to 22% of cases, respectively.124,125 The prognosis for dogs with oral osteosarcoma is better than appendicular osteosarcoma because of an apparent lower metastatic potential.119 A female sex predisposition has been reported.124 The outcome following mandibulectomy alone is variable with MSTs of 14 to 18 months and 1-year survival rates of 35% to 71%.18,124,126 In 20 dogs treated with mandibulectomy alone, the cause of death was local recurrence in 15% of dogs and metastatic disease in 35% of dogs.18 Following maxillectomy, the MST varies from 5 to 10 months with a 1-year survival rate of 17% to 27% and local tumor recurrence in 83% to 100% of dogs.19,124 The majority of dogs with maxillary osteosarcoma die as a result of local tumor recurrence with metastasis not reported in any dogs in two studies.106,125 Local tumor control is the most challenging problem and resecting oral osteosarcomas with complete surgical margins is imperative. In one study of 60 dogs with osteosarcoma of the skull, including the mandible and maxilla, the median DFI and survival times were not reached at greater than 1503 days following complete excision and significantly better than incomplete resection with a median DFI of 128 days and a MST of 199 days.127 The combination of surgery with either RT or chemotherapy did not improve the outcome in dogs with incompletely resected tumors, highlighting the necessity for an aggressive surgical approach. These results are supported by another study of 45 dogs with axial osteosarcoma in which favorable prognostic factors included complete surgical excision, mandibular location, and smaller body weight dogs.125 Chemotherapy’s role in the management of dogs with axial osteosarcoma is unknown but should be evaluated.
Cancer of the Gastrointestinal Tract
 Section A
Section A
Oral Tumors
Incidence and Risk Factors
Pathology and Natural Behavior
Malignant Melanoma
Squamous Cell Carcinoma
Fibrosarcoma
Epulides
Peripheral Odontogenic Fibroma
Acanthomatous Ameloblastoma
History and Clinical Signs
Diagnostic Techniques and Work-Up
Therapy
Radiation Therapy
Chemotherapy
Prognosis
Malignant Melanoma
Squamous Cell Carcinoma
Canine Oral Squamous Cell Carcinoma
Feline Oral Squamous Cell Carcinoma
Fibrosarcoma
Osteosarcoma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cancer of the Gastrointestinal Tract