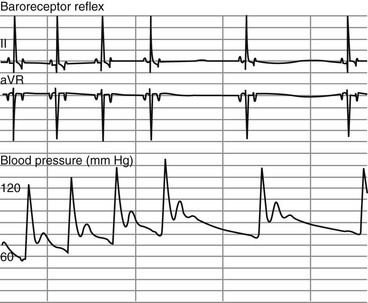
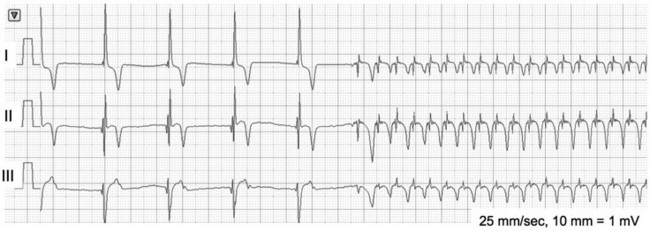
Chapter 170 A frequently observed physiologic bradyarrhythmia is the development of sinus bradycardia secondary to activation of the baroreceptor reflex. The baroreceptor reflex is an intrinsic physiologic response to an increase in systemic blood pressure that results in an elevation in cardiac vagal tone and a decrease in sympathetic tone (Figure 170-1). The baroreceptors responsible for this response are found primarily in the aortic arch and carotid sinus. Figure 170-1 Baroreceptor reflex in an anesthetized dog. Lead II and aVR surface electrocardiograms are shown on top and the arterial blood pressure tracing is shown below. The increase in systemic pressure is associated with a decrease in sinus rate. Although the cause of SSS in dogs is still unclear, mutations in cardiac sodium channels (e.g., SCN5A), connexins, and α smooth muscle heavy-chain proteins; dysfunction of ryanodine receptor 2, which comprises the calcium clock in the SA node; and SA node fibrosis have been identified in people with this disease. SSS is seen most commonly in middle-aged or older small-breed dogs, and canine breeds that are predisposed to SSS include the miniature schnauzer, West Highland white terrier, dachshund, boxer, and cocker spaniel. These breed predispositions suggest a genetic component to the cause of canine SSS. Dogs with SSS may demonstrate sinus bradycardia or a sinus rhythm with periods of sinus arrest. Periods of sinus arrest may be interrupted by junctional or ventricular escape beats or rhythms. Importantly, the escape beats and rhythms in dogs with SSS often are inadequate and delayed, which suggests concurrent dysfunction of secondary (rescue) pacemaker sites in the more distal conduction system. Dogs with SSS also commonly demonstrate a sinus rhythm with intermittent bouts of supraventricular tachycardia that are characterized by positive or negative P waves (bradycardia-tachycardia syndrome) (Figure 170-2). AV conduction disturbances ranging from first- to second-degree AVB of varying severity have been observed, but the prevalence of coexisting AVB is not known. A prolongation of QT interval and sometimes prominent T waves are seen in some dogs with SSS. Abnormal findings may not be apparent on a baseline ECG in dogs with SSS, which highlights the importance of performing 24-hour Holter monitoring or capturing the cardiac rhythm during a clinical event on a loop recorder (event monitor) for definitive diagnosis in many cases. Even if a dog does not have a collapsing episode during the time that Holter monitoring is performed, findings such as a 6- to 7-second sinus pause may strongly suggest a diagnosis of SSS. Figure 170-2 Lead I, II, and III surface electrocardiograms obtained from a 6-year-old female spayed miniature schnauzer with sick sinus syndrome. Note the initial sinus arrest with ventricular escape rhythm followed by supraventricular tachycardia initiated with negative P waves in leads I and II.
Bradyarrhythmias
Physiologic Bradyarrhythmias
Pathologic Bradyarrhythmias
Sinoatrial Node Abnormalities
Sick Sinus Syndrome (Sinus Node Dysfunction)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bradyarrhythmias
Only gold members can continue reading. Log In or Register to continue
