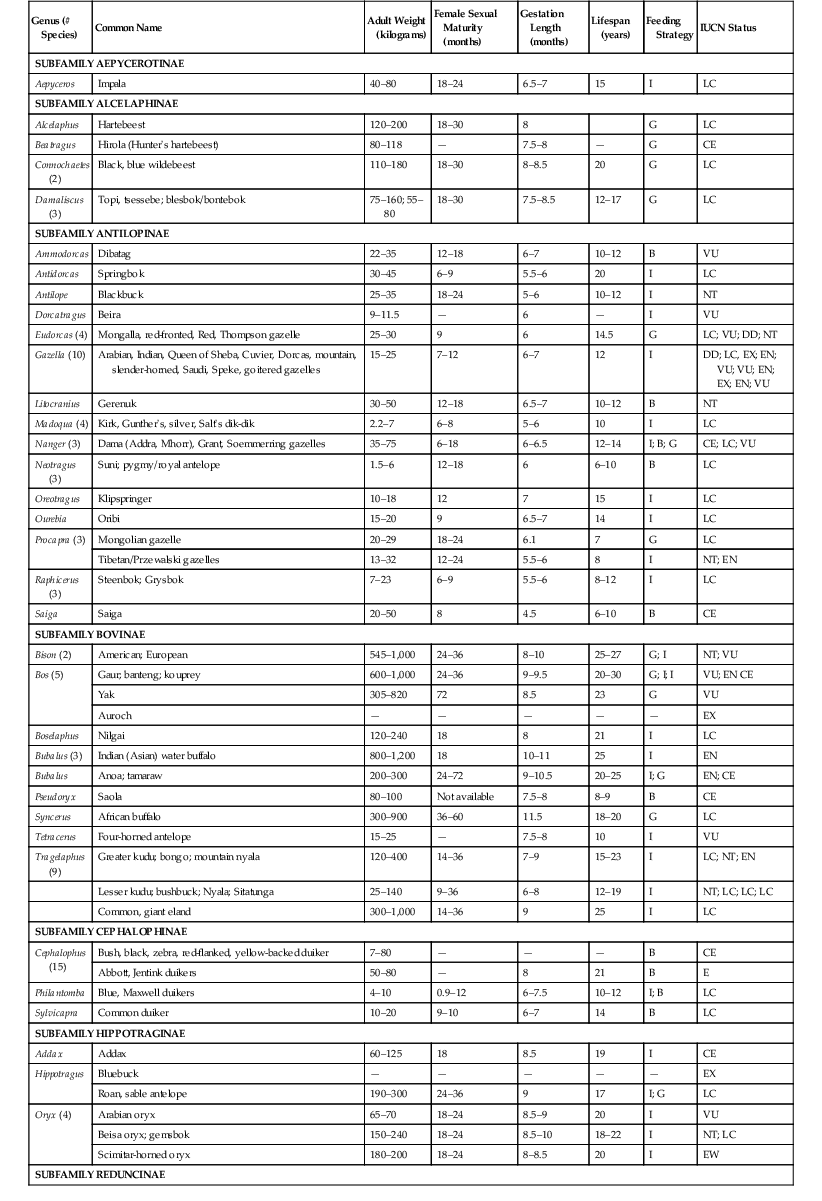
Barbara A. Wolfe Formerly classified in the order Artiodactyla, even-toed ungulates have been reclassified, on the basis of recent molecular evidence, to the order Cetartiodactyla, which includes the families Cetacea, Hippopotamidae, Camelidae, Suidae, Tayassuidae, Tragulidae, Moschidae, Cervidae, Giraffidae, Bovidae, and Antilocapridae.23 The last six of these families belong to the suborder Ruminantia. Antilocapridae consists of a single species, the pronghorn Antilocapra americana, whereas Bovidae consist of eight subfamilies: Aepycerotinae, Alcelaphinae, Antilopinae, Bovinae, Cephalophinae, Caprinae, Hippotraginae, and Reduncinae. General taxonomy and characteristics of Antilocapridae and Bovidae are listed in Table 63-1. TABLE 63-1 Taxonomy and General Characteristics of the Family Bovidae1,26,41 B, Browser; CE, critically endangered; EN, endangered; EW, extinct in the wild; EX, extinct; G, grazer; I, intermediate; LC, least concern; NT, near threatened; VU, vulnerable. Pronghorns (Antilocapra americana) are the sole species of the family Antilocapridae and are distinct from cervids and antelopes in that they possess forked horns, which are shed annually.1 Five subspecies exist: A.a. anteflexa, A.a. oregona, A.a. mexicana, A.a. peninsularis, and A.a. sonoriensis. Ranging throughout western North America from northern Mexico to southern Canada, pronghorn are found in open areas of prairies and deserts, eating primarily forbs, browse, and grasses. The Sonoran and Peninsular subspecies are considered Endangered according to federal classification. Although extremely fast runners, they are not agile jumpers, and local populations have been fragmented by fencing.57 They are extremely fractious, are prone to stress hyperthermia, and may be difficult to maintain in captivity.10 Pronghorns are fall breeders, producing twins in spring, and are the only known ungulates to exhibit multiple paternity.7 The diverse family Bovidae consists of 143 known species, ranging in size from the 3-kilogram (kg) royal antelope to the 1200-kg gaur. Bovids are found across all of mainland Africa and in 30 countries in Europe, the Middle East, and Asia. Four subfamilies exist only in the African continent; none is native to Australia or Antarctica; and only bison (Bison bison) are native to the Americas. Bovid species are characterized by the presence of horns composed of keratinized sheaths covering a bony prominence of the frontal bone, which are never shed. All males, and the females of some genera, possess horns. Only Tetracerus quadricornis, the four-horned antelope, possesses more than two horns. Most bovid horns continue to grow throughout life and are unbranched, varying in shape from short spikes to long, curved, and spiraled structures. Bovids and pronghorns are true ruminants, possessing four specialized stomach chambers. Pronghorns and other primarily grazing species have a large, stratified rumen, a smaller reticulum, a well-developed omasum, a larger abomasum, and roughly twice the relative intestinal length (25–30 times body length) of browsing species. This anatomy is adapted for digestion of large amounts of cellulose. Browsers have a smaller rumen, with evenly distributed, dense ruminal papillae, and tend to have comparatively larger salivary glands and livers compared with grazers.51 A gallbladder is usually present in bovids, and the kidneys may or may not be lobulated.10 Reproductive anatomy is similar to that of domestic cattle in most bovids, with the exception that females of some species such as the Hippotraginae demonstrate duplex uteri, in which the cervix is bifurcated, creating a physical separation between the uterine horns with no uterine body. Bovid and pronghorn species lack upper incisors and canines. The dental formula is: incisors (I) 0/3, canines (C) 0/1, premolars (P) 3/3, molars (M) 3/3, to a total of 32. Scent glands vary in size and location and may be found in the subauricular, prefrontal, forehead, submandibular, inguinal, interdigital, metacarpal, and preputial regions. Bovids have an unguligrade stance, walking on well-developed hooves on the third and fourth digits of each foot. The second and fifth digits, if present, are called dewclaws. The hooves of each species have a characteristic size and shape adaptively suited to their habitat. The 21st century has brought a grim outlook to the conservation of nondomestic bovid species. Ever-increasing human populations and their livestock encroaching on natural habitats, agriculture, transportation infrastructure, and fencing are some of the factors causing disruption of habitat and migration and are decreasing population size and genetic diversity across the taxon. In captivity, a loss of nearly 1000 spaces for antelope between 1999 and 2011 and a predicted decline in future space are reported by the Association of Zoos and Aquariums (AZA) Antelope Taxon Advisory Group.2 Of the 82 species of ungulates in AZA collections, 42% are in decline or have a negative growth rate. Few sustainable bovid populations—those that are able to persist indefinitely without supplementation—exist, either in the wild or in captivity. Over 25% of antelope species are threatened by extinction, three exist in populations of fewer than 500 individuals, and many are not represented by captive populations, all of which increases the risk of extinction.34 Recent recommendations to manage antelope in larger, less intensively managed groups have been based on (1) the difficulty of maintaining genetic diversity in small, isolated captive populations; (2) the risk of transporting animals for breeding purposes; (3) advanced methodology in genetics and population modeling; and (4) the relative success of large-landscape game ranches in population management.34,57 Pronghorns in the wild are accustomed to wide open spaces in arid regions and may tolerate wide temperature fluctuations. They often do not thrive in captivity and are compromised by high humidity, unnatural social structure, novel stimuli, and enclosed spaces. They are more likely to crawl under fencing than to jump over fencing 1.5 meters (m) or higher, but until accustomed to an enclosure, this fractious species should have visible sight barriers and minimal obstacles near fence lines to reduce trauma. A shelter that does not restrict view may be preferred by pronghorns over an enclosed barn.10 In captivity, they benefit behaviorally from frequent human proximity from a young age to maintain tractability. Pronghorns are relatively tolerant of species with which they have historically grazed (bison, elk, and deer); however, interspecific aggression has been documented.10 Housing and husbandry practices for antelope and cattle species vary widely, depending on management goals, climate, and available space. Fences and bomas may be constructed of a variety of materials and should be a minimum of 2.5 m (8 feet) in height and designed with the species size, temperament, and jumping ability in mind. Fences with sight barriers are beneficial in management areas to prevent trauma and between adjacent enclosures to prevent aggression and fence damage. Electric wires may be useful for keeping animals away from fence lines but may also present an entrapment and trauma risk through horn entanglement. Space and temperature recommendations are listed in Table 63-2. Some species may experience regular hoof problems when housed on substrates to which they are not adapted. For example, hoof abscesses may be common in desert species exposed to muddy conditions for prolonged periods. Indoor substrates should be considered with regard to hoof hardness and wear to minimize trimming and hoof lesions. TABLE 63-2 Recommended Housing Conditions for Selected Species of Antilocapridae and Bovidae* * Personal communication: D. Beetem and M. Fischer, AZA Antelope Taxon Advisory Group. Shelter conditions should take into account the natural history of the species and ambient conditions. Desert-adapted animals may tolerate high temperatures (≥38° C [100° F]) provided adequate water and shade are available, but most desert and tropical species need to be housed indoors during the cold season in colder regions. Frostbite and ear tip loss are frequently seen at temperatures below 9° C (15° F). Forest and cold-adapted or altitude-adapted species may require more shade during the warm season, and shelters for all species should provide cover and windbreaks facing away from prevailing winds.10 Forest species such as duikers will benefit from plantings or other structures that support hiding behaviors. Indoor housing should provide temperatures between 10° C and 27° C (50° F–80° F), with adequate ventilation to control humidity and reduce ammonia concentrations. Maternal rejection is not uncommon in captive nondomestic ruminants. If a calf cannot be raised by its dam, hand rearing is labor intensive and requires early intervention to provide the calf with the best chance of survival. Neonatal ruminants acquire immune protection passively through ingestion of maternal colostral immunoglobulins (IgG) in the first 24 to 48 hours of life. The calf’s ability to absorb antibodies ends approximately 24 hours after the first meal,15 and failure of passive transfer (FPT) occurs when inadequate levels of IgG are absorbed during this period. Even calves nursing from their dams may experience FPT by ingesting colostrum in inadequate volume or low IgG content, and FPT calves are highly susceptible to disease, often into the postweaning period. The prophylactic use of parenteral antimicrobials in calves with FPT should be considered but must be combined with colostrum replacement and management practices that minimize pathogen exposure. The first choice for colostrum replacement should be oral administration of fresh or frozen intraspecific colostrum, followed by low-temperature pasteurized cow’s colostrum, commercial freeze-dried cow’s colostrum replacer, and commercial bovine plasma. Feed calves 10% of their body weight in colostrum, or 5 grams per kilogram (g/kg) colostrum replacer, over the first 24 to 48 hours. Tests for FPT are readily available.56 If passive transfer is inadequate after 48 hours, calves may receive parenteral conspecific plasma or commercial bovine plasma (20–40 milliliters per kilogram [mL/kg]). Milk composition varies considerably among species, and a formula’s composition should mimic that of the dam’s milk in protein, carbohydrate, fat, and total solids. Goat’s milk is a good choice for many species, alone or in combination with a milk replacer. However, milk may be low in vitamin E, zinc, copper, and iron, necessitating vitamin and mineral supplementation. Probiotics may also be beneficial in establishing a healthy rumen flora. Calves should receive 8% to 15% of their body weight in formula every 24 hours, divided into four to six feedings per day. Resources are available to assist with milk replacer formulation and methods of feeding.48,59 In the first 2 to 3 weeks of a ruminant’s life, milk digestion occurs in the abomasum and small intestine. Milk deposited into the nonfunctional rumenoreticulum during this period is not digested and may lead to rumenitis and septicemia.15 For this reason, tube feeding or force feeding a calf with a poor suckle response may be harmful. Closure of the esophageal groove is stimulated by suckling and normally prevents this deposition. If tube feeding is necessary in the first weeks of life, a tube passed to the mid-esophagus may stimulate swallowing and closure of the groove. Additionally, oral administration of 10% sodium bicarbonate or 2% to 5% copper sulfate prior to milk feeding may facilitate groove closure for several minutes. By 2 to 3 weeks, the ruminal papillae are stimulated, and the calf begins to take in small amounts of dry feed. Changes in gastrointestinal (GI) flora occur throughout this period and until weaning (around 4 months), often manifesting as changes in fecal consistency. Many of the common health problems of nondomestic ruminants have recently been associated with direct or indirect dietary causes.11 Chronic weight loss, rumen acidosis or rumenitis, laminitis, hoof overgrowth, and periodontal disease have all been attributed, at least in part, to historical feeding of all exotic bovids diets designed for domestic cattle. Cattle are grazing species, but many of the nondomestic Bovidae are intermediate or browse feeders. Browsers are adapted to eating the leaves and twigs of woody plants, intermediate feeders to both browse and grasses, and grazers to consuming grasses (see Table 63-1). All of these forages are fermented by the microbes of the ruminoreticulum, which produce fatty acids, providing energy to the ruminant. The grazer rumen is adapted for fermenting the high cellulose content in grasses through prolonged fermentation and particle retention, compared with the smaller, less muscular browser rumen. Consequently, browsers tend to eat less hay and are often fed higher amounts of pelleted concentrate to compensate for low hay consumption. High levels of easily digested carbohydrates such as starch and sugar, often found in pelleted concentrates, are too rapidly fermented in the rumen, leading to rumen acidosis. The ideal rumen pH range is 6.7 ± 0.5, and variation from this results in disturbance of the microflora. Altered and depleted rumen flora decrease the energy delivered to the ruminant, which results in weight loss. In response, many of these animals will receive increased amounts of concentrate, which compounds the problem. Rumen acidosis and resulting rumenitis may also lead to systemic acidosis, mineral imbalances, and laminitis or abnormal hoof growth. Appropriately formulated pelleted feed for browsers should be based on a high-fiber forage meal such as aspen, alfalfa, soy or sunflower hulls, or cellulose powder, and should contain (1) a high-pectin, low-sugar energy source such as beet pulp; and (2) limited amounts of grain and corn.11 A low-starch, high-fiber pelleted diet that meets these criteria has been recently formulated for browsing and intermediate ruminants (Wild Herbivore and Wild Herbivore Plus; Mazuri Zoo Feeds, PMI Feeds, St. Louis, MO). The recommended feed intake is 1.5% to 2.5% of body weight per day in addition to ad libitum grass or legume hay, and supplementation with natural nontoxic browse is recommended for all browsers. Grazers are generally fed commercial herbivore pelleted concentrate diet containing 12% to 18% protein and 16% to 25% acid detergent fiber at approximately 1% body weight per day in addition to hay. Salt blocks should be available at all times, and trace mineral salt blocks should be available to herds primarily on pasture or not receiving trace mineral balanced concentrates. Adult bovids experiencing negative energy balance and weight loss from illness may require nutritional support during treatment. Commercially available products useful for boosting caloric intake include Low Odor MEGALAC Rumen Bypass Fat (Arm & Hammer, Church & Dwight Co., Inc., Princeton, NJ) and Wild Herbivore Boost (Mazuri Zoo Feeds, PMI Feeds, St. Louis, MO). These compromised animals may also benefit from transfaunation with rumen contents collected from a healthy conspecific. Tube feeding ruminants may be challenging because of the consistency and volume of feed they require; a commercial tube-feeding formula is available for herbivores (Critical Care, Oxbow Pet Products, Murdock, NB).10 Restraint is an important aspect of medical practice in nondomestic ruminants, requiring careful planning and experience.21 Because of the size and fractious nature of the species, most wild bovids require restraint for any type of physical examination for the safety of both the animal and the handlers. Capture and restraint of animals in the wild is conducted by a multitude of means, including drive nets, drop nets, net guns, bomas, chutes, traps, and remote injection. Some of these procedures require helicopters and specialized training and are conducted by full-time capture professionals. In zoologic settings, capture and restraint capabilities depend on the facilities and expertise available, and each procedure conducted must be planned with the animal, handler ability, and equipment in mind. Restraint may be classified as physical, behavioral, and chemical, and many procedures in these taxa will require some combination of all three methods. Planning for any restraint procedure should take into account the goal, the conditions (e.g., restraint type and capability, ambient temperature, footing, enclosure size), and the temperament of the animal.9 Restraint planning should also include plans for alternative physical and chemical restraint and emergency release in case of injury, failure of behavioral compliance, or signs of severe distress in the animal. Safety of the personnel and the patient are the primary considerations. Any manipulation of bovids and pronghorns may result in extreme panic, self-injury, capture myopathy (see Noninfectious Disease), and even sudden death, necessitating efficiency of time and force used to achieve the goal of restraint. Continuous development of improved behavioral management techniques in captive settings has allowed for procedures that once required anesthesia to be performed with minimal restraint. Some species accustomed to human proximity may be managed to a degree with little or no physical restraint through training of specific behaviors such as placing feet for hoof trimming. Desensitization and operant conditioning may be used to train animals to move calmly to a restraint area, enter a chute or crate, lie down, or tolerate minor procedures, but special attention to the explosive nature of many bovids requires slow, deliberate movements, quiet conditions, and safety precautions. Movement may be aided by careful design and planning of stalls, doors, hallways and chute approach, and the use of baffle boards may protect the handler and assist in movement. Smaller species may be manually restrained by experienced handlers for brief procedures or induction of anesthesia after being caught by hand or with hoop nets. Restraint should be initiated on an isolated animal in a darkened, quiet, obstacle-free enclosure that is small enough to minimize the risk of injury to the animal but large enough to allow free movement and escape of the handlers. A stall with padded walls and hay or straw floor substrate is optimal for animal safety. A minimum of two handlers should perform the capture, depending on the size and temperament of the animal, and the handlers should wear protective clothing, footwear, eyewear and gloves. Hand restraint in bovids under 5 kg may be accomplished by a single handler by quickly lifting the animal, supporting the abdomen and spine against the handler’s body, and restraining the head and legs. Restraint of medium-sized animals may be initiated by catching the head and quickly pushing the animal against a wall, pad, or floor with head restraint and placing a knee under the flank, with a second handler restraining the rear legs. Horns may be used for restraint of most species, but young animals or those with thin horns may be prone to horn avulsion. The head and legs should be tightly restrained to prevent the use of horns and hooves, and placement of a hand or a pad between the hocks may help to prevent self-trauma. The duration of physical restraint should be minimized to prevent distress and hyperthermia, and a clear airway and adequate chest excursion should be ensured at all times. Use of a blindfold may reduce struggling. If an animal is to be restrained repeatedly, short pieces of hosing placed on the horn tips improves safety for the handlers. Release should be coordinated such that handlers act quickly and in concert, directing the animal toward a clear path and to an open area that provides the patient with a sense of refuge and minimizes the risk of injury. The development of sophisticated chute systems for hoof stock has allowed for the handling of entire herds of nondomestic bovids rapidly and without chemical restraint.25 Procedures such as venipuncture, vaccination, tuberculin testing, physical examination, treatment of minor conditions, hoof trimming, and reproductive procedures may be conducted without chemical restraint in an effectively designed chute system. In designing a restraint, considerations such as maneuvering animals from a pen into the chute, the ability to stop and sort animals within the system, the inclusion of movable rear walls for pushing resistant animals, the presence of a scale within the system, and multiple exit routes may be critical to creating a process that is efficient and safe, minimizing time and stress to the animals. Desensitizing animals to any chute system by incorporating movement through the system into the animals’ regular routine may greatly facilitate restraint. Many facilities housing large numbers of bovid and equid species are now using chute systems to facilitate preventive medicine, reproductive management, research, and extended treatment of animals, while alleviating the need for repeated chemical restraint. Mechanical restraints are of three basic types: (1) box chutes or stanchions, (2) drop-floor chutes, and (3) crushes or squeeze chutes. Box chutes are the simplest and least expensive of the three, consisting of a simple pass-through enclosure with front and rear sliding barriers to retain the animal temporarily. Rear entry or exit restraints are not recommended for nondomestic bovids, as many of these animals are reluctant to enter an area with no perceived exit. Drop-floor chutes are available commercially and consist of a ramp leading to, or a recessed area underneath, an adjustable V-shaped chute with sliding front and rear doors. When the animal is secured in the chute, the floor is dropped such that the animal is suspended by the hips and shoulders (Tamer Drop Floor Chute, Fauna Research, Inc., Red Hook, NY). Most bovids will refrain from struggling without foot purchase, allowing short procedures to be conducted. Width settings for each animal should be carefully established with drop-floor chutes to provide proper restraint, while not overly restricting the abdomen and chest, and duration of procedures should be limited to a few minutes. Drop-floor chutes may be portable or permanently installed. Squeeze chutes vary from simple adaptations to an aisle way to highly adjustable hydraulic systems. Squeeze chutes developed for domestic cattle have been adapted to larger nondomestic bovids such as wild cattle and bison by adding “crash” gates—barred swinging front doors to prevent bypass of the head gate. Hydraulic squeeze chutes, available commercially (e.g., Hydraulic Tamer, Fauna Research, Inc., Red Hook, NY), provide the most flexible and rapid manipulation of large numbers of animals, as they are adjusted remotely while the animal is in the device. The padded walls of this restraint device are moved hydraulically to apply pressure to the hips and shoulders of the animal and may lift—achieving a result similar to a drop-floor chute—or close down over the animal to provide a darkened enclosed space. Doors installed in the restraint walls provide access for examination, treatment, and venipuncture. When physical and behavioral restraints are inadequate to maintain control of an animal for the length of time or invasive nature of the desired procedure, chemical restraint is necessary as an adjunct or sole method of restraint. Chemical restraint is commonly necessary in the management of nondomestic bovids and carries significant risks because of the fractious nature of the species, the difficulty of drug delivery, and the unique biology of the taxon. Veterinary practitioners caring for nondomestic Bovidae are constantly evaluating and improving protocols for sedation and anesthesia, as sensitivity to different anesthetics among nondomestic bovids tend to be species-specific. Chemical restraint in this taxon has been the subject of many reviews.3,10,13,29,53 Chemical restraint may be accomplished by two means: tranquilization and general anesthesia. Increasingly, neuroleptics of the butyrophenone and phenothiazine families have been used to attenuate the stress response in nondomestic ruminants undergoing intensive management for capture, translocation, isolation, or adaptation to environmental changes.10,16,44 Licensed for use in humans as antipsychotic agents, these drugs act by blocking D2 dopamine receptors, thereby producing a state of lucid relief from anxiety. Most of the longer-acting formulations have an onset of action of 1 to 3 days, necessitating the addition of short-acting neuroleptics to produce immediate and long-term tranquilization. Table 63-3 lists common formulations and their onset and duration of action, and Table 63-4 lists suggested doses of neuroleptic drugs for tranquilization of pronghorns and bovids. Recommended dosages per kilogram of body weight are inversely proportional to body size. It should be noted that neuroleptic use may improve the ease of captive management of smaller hoof stock by increasing flight distance, but reducing the fear response may have the opposite effect in larger, more aggressive species, resulting in reduced avoidance behavior, reluctance to move, and aggression. Overdosing is known to produce behavioral side effects such as tardive dyskinesia, including abnormal facial and tongue posture, head pressing and other unusual movement patterns, and anorexia. Treatment of clinical signs of overdosing may be accomplished using low doses of xylazine, diazepam, or diphenhydramine.44 TABLE 63-3 Onset and Duration of Action of Selected Neuroleptics Used in Bovidae16,44 IM, Intramuscularly; IV, intravenously. TABLE 63-4 Suggested Doses of Selected Neuroleptics Used in Adult Bovidae16,44 Note: All doses are intended for intramuscular use unless indicated. IM, Intramuscularly; IV, intravenously. General anesthesia is induced parenterally by intramuscular or intravenous routes. Intravenous administration may be performed on manually or mechanically restrained animals and provides the advantage of decreased induction and recovery time. However, in larger animals, intravenous administration of a mechanically restrained animal may result in difficulty in removing the rapidly-induced animal from the restraint device. In general, intramuscular induction of an animal with an appropriate regimen in a darkened, quiet enclosure provides a relatively rapid and smooth induction and may minimize the need for anesthetic supplementation because of longer duration of action. Administration of anesthetics intramuscularly (IM) may be conducted by hand, pole syringe, or projectile dart. The use of darts and remote injection systems has been previously reviewed27 and requires careful planning and experience. Planning an anesthetic event should include considerations such as environmental temperatures, approach, enclosure size, obstacles, and the presence of other animals. Failure to deliver an appropriate dose accurately and quickly to an animal in the wild or in a large enclosure may result in excessive running or pacing injury, capture stress or myopathy, and death. Muscle masses of the rump, shoulder, and neck are preferred sites of remote injection. Failure of induction within an appropriate time (usually 5 to 15 minutes, depending on the regimen) may be caused by many factors, including dart failure, inappropriate dosing, and operator error. A partially anesthetized animal is susceptible to capture myopathy, so a decision to repeat induction should be made within 20 minutes of initial dart placement. Supplementation by remote means generally requires a full anesthetic dose, as the animal may be rapidly metabolizing the anesthetic because of stress and activity. Many anesthetic regimens have been described for pronghorns and bovids,3,10,29 ,53 and these regimens generally consist of ultrapotent narcotics (carfentanil, etorphine, thiafentanil), with or without sedatives or tranquilizers (α2-agonists, butyrophenones), dissociative cyclohexamines (ketamine or tiletamine), or both. The use of the mixed opioid agonist–antagonist butorphanol tartrate as a component of anesthetic cocktails has been recently reviewed.6 Historically, the ultrapotent opioid (narcotic) agents carfentanil citrate, etorphine, and thiafentanil oxalate have been the primary components of anesthetic cocktails used in nondomestic bovid species for many reasons: (1) Opioid agents have a relatively wide margin of safety for the patient and are highly potent, allowing for small volumes to be contained in remote delivery darts; (2) they are rapid acting and provide an efficient induction and minimize the risk of hyperthermia; and (3) they are reversible. However, ultrapotent opioids are associated with significant side effects such as suppression of respiration and GI motility, poor muscle relaxation, and renarcotization46 and carry a risk to human safety, as the lethal human dose of some agents is as low as 20 micrograms (µg).29 Ultrapotent opioids are Schedule II substances controlled by the Drug Enforcement Agency and thereby require the practitioner to possess special licensing and to follow defined possession, storage, and recordkeeping guidelines. Safety protocols and special training for handling narcotics and exposure emergencies must be maintained for all staff involved in ultrapotent narcotic procedures. Carfentanil citrate is the most potent of the three agents. Etorphine and thiafentanil oxalate may produce desirable effects such as improved muscle relaxation and decreased respiratory suppression in some species. Opioids, particularly carfentanil, may produce general anesthesia when administered alone; however, combination with other agents may reduce the narcotic dose, ease induction and recovery, increase muscle relaxation, and decrease respiratory suppression. The α2-agonists reported in bovid anesthetic regimens include xylazine, detomidine, and medetomidine. In general, bovids are more sensitive to the α2-agonists compared with equids, and these agents may improve muscle relaxation, decrease narcotic doses, and ease induction and recovery. In smaller bovids and pronghorns, these drugs may be combined with cyclohexamines, benzodiazepines, butorphanol, or a combination, eliminating the need for an ultrapotent opioid. α2-agonists, however, require 20 minutes to reach full effect when administered IM; approaching a recumbent animal prior to this time may result in spontaneous recovery and the need to re-administer the induction dose. α2-agonists—particularly medetomidine—cause peripheral vasoconstriction, which may result in hypertension, bradycardia, poor mucous membrane color, and second-degree atrioventricular blocks. Use of parasympathomimetics such as atropine or glycopyrrolate to increase heart rate is controversial, as the resulting increased cardiac output may exacerbate hypertension. Medetomidine is available in highly concentrated formulations (Wildlife Pharmaceuticals, Inc., Fort Collins, CO), improving its usefulness for remote delivery in large species. Dissociative anesthetics include the cyclohexamines ketamine hydrochloride and tiletamine (which is formulated in combination with the benzodiazepine zolazepam). These drugs are rapid acting, carry a high margin of safety, and are often used as adjuncts or supplemental anesthetics either intravenously (IV) or IM at 0.3 to 1.0 mg/kg. However, cyclohexamines are not reversible and thereby may affect duration of and recovery from anesthesia. When ketamine is used as a supplement, antagonism of reversible anesthetics should be delayed until 20 minutes after ketamine administration. This allows for ketamine metabolism and may prevent stormy recovery. Tiletamine, particularly when used without reversal of the zolazepam component (see Table 63-6), may be associated with prolonged recovery. A partial list of suggested regimens for general anesthesia of bovids and pronghorns by species is presented in Table 63-5, and suggested reversal agents and doses are listed in Table 63-6. TABLE 63-5 Combinations of Chemical Restraint Agents Used for Induction of Anesthesia in Selected Species of Antilocapridae and Bovidae*3,8,10,29,31,53
Bovidae (Except Sheep and Goats) and Antilocapridae
General Biology
Genus (# Species)
Common Name
Adult Weight (kilograms)
Female Sexual Maturity (months)
Gestation Length (months)
Lifespan (years)
Feeding Strategy
IUCN Status
SUBFAMILY AEPYCEROTINAE
Aepyceros
Impala
40–80
18–24
6.5–7
15
I
LC
SUBFAMILY ALCELAPHINAE
Alcelaphus
Hartebeest
120–200
18–30
8
G
LC
Beatragus
Hirola (Hunter’s hartebeest)
80–118
—
7.5–8
—
G
CE
Connochaetes (2)
Black, blue wildebeest
110–180
18–30
8–8.5
20
G
LC
Damaliscus (3)
Topi, tsessebe; blesbok/bontebok
75–160; 55–80
18–30
7.5–8.5
12–17
G
LC
SUBFAMILY ANTILOPINAE
Ammodorcas
Dibatag
22–35
12–18
6–7
10–12
B
VU
Antidorcas
Springbok
30–45
6–9
5.5–6
20
I
LC
Antilope
Blackbuck
25–35
18–24
5–6
10–12
I
NT
Dorcatragus
Beira
9–11.5
—
6
—
I
VU
Eudorcas (4)
Mongalla, red-fronted, Red, Thompson gazelle
25–30
9
6
14.5
G
LC; VU; DD; NT
Gazella (10)
Arabian, Indian, Queen of Sheba, Cuvier, Dorcas, mountain, slender-horned, Saudi, Speke, goitered gazelles
15–25
7–12
6–7
12
I
DD; LC, EX; EN; VU; VU; EN; EX; EN; VU
Litocranius
Gerenuk
30–50
12–18
6.5–7
10–12
B
NT
Madoqua (4)
Kirk, Gunther’s, silver, Salt’s dik-dik
2.2–7
6–8
5–6
10
I
LC
Nanger (3)
Dama (Addra, Mhorr), Grant, Soemmerring gazelles
35–75
6–18
6–6.5
12–14
I; B; G
CE; LC; VU
Neotragus (3)
Suni; pygmy/royal antelope
1.5–6
12–18
6
6–10
B
LC
Oreotragus
Klipspringer
10–18
12
7
15
I
LC
Ourebia
Oribi
15–20
9
6.5–7
14
I
LC
Procapra (3)
Mongolian gazelle
20–29
18–24
6.1
7
G
LC
Tibetan/Przewalski gazelles
13–32
12–24
5.5–6
8
I
NT; EN
Raphicerus (3)
Steenbok; Grysbok
7–23
6–9
5.5–6
8–12
I
LC
Saiga
Saiga
20–50
8
4.5
6–10
B
CE
SUBFAMILY BOVINAE
Bison (2)
American; European
545–1,000
24–36
8–10
25–27
G; I
NT; VU
Bos (5)
Gaur; banteng; kouprey
600–1,000
24–36
9–9.5
20–30
G; I; I
VU; EN CE
Yak
305–820
72
8.5
23
G
VU
Auroch
—
—
—
—
—
EX
Boselaphus
Nilgai
120–240
18
8
21
I
LC
Bubalus (3)
Indian (Asian) water buffalo
800–1,200
18
10–11
25
I
EN
Bubalus
Anoa; tamaraw
200–300
24–72
9–10.5
20–25
I; G
EN; CE
Pseudoryx
Saola
80–100
Not available
7.5–8
8–9
B
CE
Syncerus
African buffalo
300–900
36–60
11.5
18–20
G
LC
Tetracerus
Four-horned antelope
15–25
—
7.5–8
10
I
VU
Tragelaphus (9)
Greater kudu; bongo; mountain nyala
120–400
14–36
7–9
15–23
I
LC; NT; EN
Lesser kudu; bushbuck; Nyala; Sitatunga
25–140
9–36
6–8
12–19
I
NT; LC; LC; LC
Common, giant eland
300–1,000
14–36
9
25
I
LC
SUBFAMILY CEPHALOPHINAE
Cephalophus (15)
Bush, black, zebra, red-flanked, yellow-backed duiker
7–80
—
—
—
B
CE
Abbott, Jentink duikers
50–80
—
8
21
B
E
Philantomba
Blue, Maxwell duikers
4–10
0.9–12
6–7.5
10–12
I; B
LC
Sylvicapra
Common duiker
10–20
9–10
6–7
14
B
LC
SUBFAMILY HIPPOTRAGINAE
Addax
Addax
60–125
18
8.5
19
I
CE
Hippotragus
Bluebuck
—
—
—
—
—
EX
Roan, sable antelope
190–300
24–36
9
17
I; G
LC
Oryx (4)
Arabian oryx
65–70
18–24
8.5–9
20
I
VU
Beisa oryx; gemsbok
150–240
18–24
8.5–10
18–22
I
NT; LC
Scimitar-horned oryx
180–200
18–24
8–8.5
20
I
EW
SUBFAMILY REDUNCINAE
Kobus (5)
Kob; red lechwe; Nile lechwe; Puku
50–130
18–24
7–9
15–21
G
LC; LC; EN; NT
Waterbuck
150–250
12–16
8.5–9
18
I
LC
Pelea
Rhebok
18–30
14
7
8–10
I
LC
Redunca (3)
Southern, mountain, Bohor reedbuck
40–95
12
7.75
16
G
LC
Antilocapridae
Bovidae
Unique Anatomy
Management and Husbandry
Population Management
Special Housing Requirements
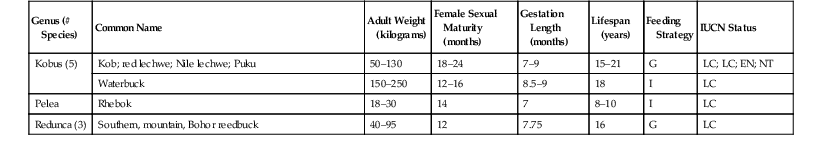
Common Name
Native Range
Native Climate
Minimum Barrier Height (feet)
Minimum Exhibit Size
(square feet)
Minimum Holding Size
(square feet)
Temperature
(° F)
Pronghorn
Not available
Temperate
8
600
200
45–100
SUBFAMILY AEPYCEROTINAE
Impala
African
Tropical
8
600
200
45–100
SUBFAMILY ALCELAPHINAE
Hartebeest, wildebeest, bontebok, blesbok, topi
African
Tropical
8
600
200
45–100
SUBFAMILY ANTILOPINAE
Dik-dik, royal antelope, steenbok, klipspringer
African
Tropical
8
200
140
45–100
Suni
African
Arid
8
200
140
45–100
Addra, Mhorr, Dorcas, Speke gazelle
African
Arid
8
400
92
45–100
Grant, Thomson’s, slender-horned, red-fronted,
Soemmerring gazelle
African
Tropical
8
400
92
45–100
Saudi goitered gazelle
Asian
Arid or tropical
8
400
92
45–100
Cuvier gazelle
African
Arid
8
600
92
45–100
Springbok
African
Arid
8
400
140
45–100
Gerenuk
African
Arid
8
400
140
60–100
Blackbuck
Asian
Tropical
8
400
140
45–100
SUBFAMILY BOVINAE
Common, giant eland
African
Tropical
10
600
200
45–100
Lowland nyala
Eastern bongo
Lesser, greater kudu
Sitatunga
African
Tropical
8
600
200
45–100
Nilgai
Asian
Tropical
8
600
200
45–100
Bushbuck
African
Tropical
8
400
140
45–100
SUBFAMILY CEPHALOPHINAE
Crowned, Maxwell blue, red-flanked duiker
African
Tropical
8
200
140
45–100
Bay, black duiker
African
Tropical
8
400
140
45–100
Yellow-backed duiker
African
Tropical
8
600
200
45–100
SUBFAMILY HIPPOTRAGINAE
Roan, sable antelope
African
Tropical
8
600
200
45–100
Addax, gemsbok scimitar-horned, Arabian oryx
African
Arid
8
600
200
45–100
Beisa, fringe-eared oryx
African
Arid or tropical
8
600
200
45–100
SUBFAMILY REDUNCINAE
Common, Defassa waterbuck
Uganda kob red, Nile lechwe
African
Tropical
8
600
200
45–100
Rhebok
African
Tropical
8
400
140
45–100
Nutrition
Feeding the Neonate
Feeding the Adult
Restraint and Handling
Behavioral Restraint
Physical Restraint
Manual Capture and Restraint
Mechanical Restraint
Chemical Restraint
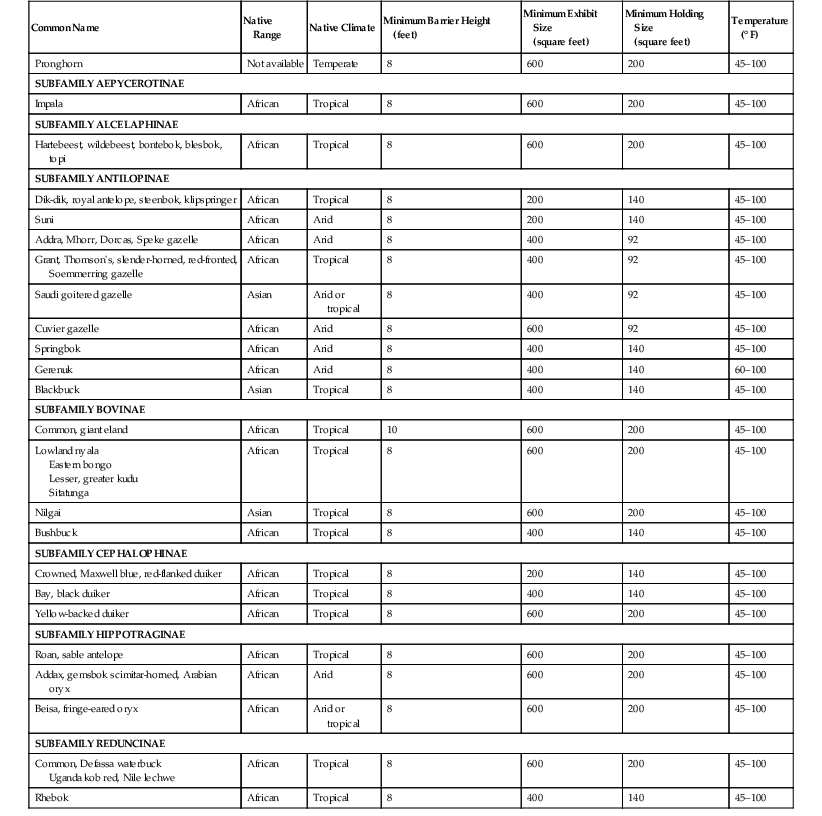
Tranquilization and Sedation
Drug
Route
Onset
Duration
Azaperone
IV
<10 minutes
≤6 hours
IM
30 minutes
≤6 hours
Haloperidol lactate
IV
<5 minutes
≤8 hours
IM
<15 minutes
8–18 hours
Zuclopenthixol acetate
IM
1 hour
3–4 days
Zuclopenthixol decanoate
IM
1 week
10–21 days
Haloperidol decanoate
IM
2–3 days
21–30 days
Perphenazine enanthate
IM
hours
7–10 days
Pipothiazine palmitate
IM
2–3 days
21–28 days
Fluphenazine decanoate
IM
3 days
21–28 days

Family
Azaperone
Haloperidol lactate
(IM or IV)
Zuclopenthixol acetate
Perphenazine enanthate
Pipotiazine palmitate
Fluphenazine decanoate
Aepycerotinae
0.5
0.2–0.5
1.0
1.0
4.0–4.5
0.5–1.0
Alcelaphinae
0.3–1.0
0.05–0.01
1.0
0.25–1.0
2.0–4.0
0.25–0.5
Antilopinae
0.25–0.5
0.1–1.0
2.0–5.0
1.0–5.0
1.0–2.5
0.5–1.0
Bovinae
0.1–0.5
0.025–0.2
0.3–2.0
0.1–1.0
0.1–1.0
0.25–0.5
Cephalophinae
0.5–1.0
0.25–0.5
—
2.0–5.0
—
—
Hippotraginae
0.5
0.1–0.2
0.5–1.0
0.5–1.0
0.25–0.5
0.25–0.5
Reduncinae
0.5–1.0
0.1–0.2
1.0–2.0
1.0
0.3–2.0
0.25–0.5
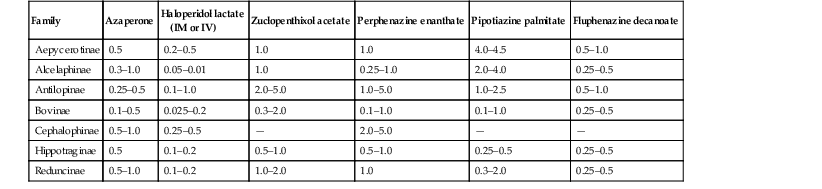
Induction of General Anesthesia
CARF
ETOR
THIA
XYL
MED
DET
KET/AZAP
ANTILOCAPRIDAE
Pronghorn
0.05
1.0
0.1
1.0
0.3
5 K
0.1
0.5
AEPYCEROTINAE
Impala
0.02–0.03
± 0.1–0.2
0.08–0.10
0.1–0.3
0.08
0.04
0.5–0.1
Or 0.025–0.05
0.20–0.25
3–5 K
ALCELAPHINAE
Hartebeest
0.01
0.15
Wildebeest
0.008
0.08
0.03
0.1
Bontebok/blesbok
0.015–0.025
0.2–0.35
1.5–2.5 K
0.020–0.025
0.2–0.3
0.2–0.3 K
0.03
0.5 A
0.05–0.09
1.0–1.3 K
Tsessebe
0.03
0.1
0.3 A
ANTILOPINAE
Springbok
0.03
0.05–0.10
0.15–0.25
0.5
9.0 K
Blackbuck
0.05
0.1
0.25
2.0 K
Addra gazelle
0.015–0.02
±0.20
0.03–0.06
0.06–0.10
1.8–4 K
Slender-horned gazelle
0.03–0.05
0.10–0.25
Grant gazelle
0.035
Thomson’s gazelle
0.02–0.03
0.05–0.07
0.2–0.45
0.2–0.45 K
Gerenuk
0.06–0. 07
1.5–2.0 K
0.04
3.5 K
Dik-dik
0.01
0.4
Suni
0.01
0.4
0.2–0.4
15 K
Klipspringer
0.01
0.4
0.05
0.16
2.1 K
Saiga
0.05–0.1
BOVINAE
Bison
0.004–0.008
0.05–0.10
0.01
0.05
0.5–1
4 K
0.05–0.08
1.5–2.5 K
Gaur, gayal, banteng
0.006–0.01
0.1–0.2
0.01–0.02
Nilgai
0.02
0.03
Anoa
0.008–0.012
0.06–0.12
African buffalo
0.005
0.05
0.015
0.1–0.15
0.01–0.025
±0.05–0.1
Giant eland
0.008
0.03
0.005–0.007
0.05–0.10
Common eland
0.01–0.016
0.15–0.20
0.02
0.40
0.03–0.07
0.1
0.8
4 K
Nyala
0.015–0.025
0.10–0.25
0.04
0.30
0.045
0.05
3–4 K
0.08
0.3
Sitatunga
0.04
0.3
Tibetan yak
0.02
0.15
Bongo
0.01–0.025
0.1–0.25
0.01
0.15–0.2
0.5 K
0.02–0.05
0.05–0.15
Greater kudu
0.02–0.25
0.2–0.25
0.02–0.03
0.2–0.3
0.05
0.25
CEPHALOPHINAE
Maxwell duiker
0.025
0.02
1
0.2–0.3
15–25 K
Blue duiker
0.01
0.40
0.3
15 K
0.2
2.2 K
Yellow-backed duiker
0.02–0.03
0.2
0.02
1
Common duiker
0.01–0.02
0.5
HIPPOTRAGINAE
Addax
0.025
± 0.15–0.25
0.03–0.04
0.02
0.05–0.06
1.0 K
Roan
0.015–0.02
0.15–0.2
0.025
0.15–0.25
0.01–0.02
0.005–0.006
0.3–0.6 K
Sable
0.015–0.02
0.15–0.2
0.015–0.025
0.1–0.2
±0.15–0.2 K
0.03
0.1–0.2
Scimitar-horned oryx
0.015–0.03
0.15–0.3
0.025
0.15
0.05
0.005
Gemsbok
0.01–0.02
0.1–0.2
0.03
0.25
0.015
0.15–0.25
0.15–0.25 K
0.02–0.04
0.02–0.04
1.0 K
Arabian oryx
0.03–0.04
0.25
0.03–0.04
0.3
0.04
0.005
0.045–0.05
0.045–0.05
0.03–0.06
1.2–2 K
REDUNCINAE
Waterbuck
0.01–0.025
0.10–0.25
0.03
0.25
0.03–0.05
0.2
Red lechwe
0.02
0.01
0.1
0.02–0.05
0.1–0.4
0.1–0.4 K
Nile lechwe
0.02
0.25
1.0–2.0 K
0.02
1.5–3.0 K
Uganda kob
0.035
0.35
Reedbuck
0.05–0.07
0.2
±0.1 A
Rhebok
0.01
0.4
0.01
0.4
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


